Deposition Date
2014-06-09
Release Date
2015-01-14
Last Version Date
2024-02-28
Entry Detail
PDB ID:
4QKR
Keywords:
Title:
Crystal Structure of 6xTyr/PV2: de novo designed beta-trefoil architecture with symmetric primary structure (L22Y/L44Y/L64Y/L85Y/L108Y/L132Y, Primitive Version 2)
Biological Source:
Host Organism:
Method Details:
Experimental Method:
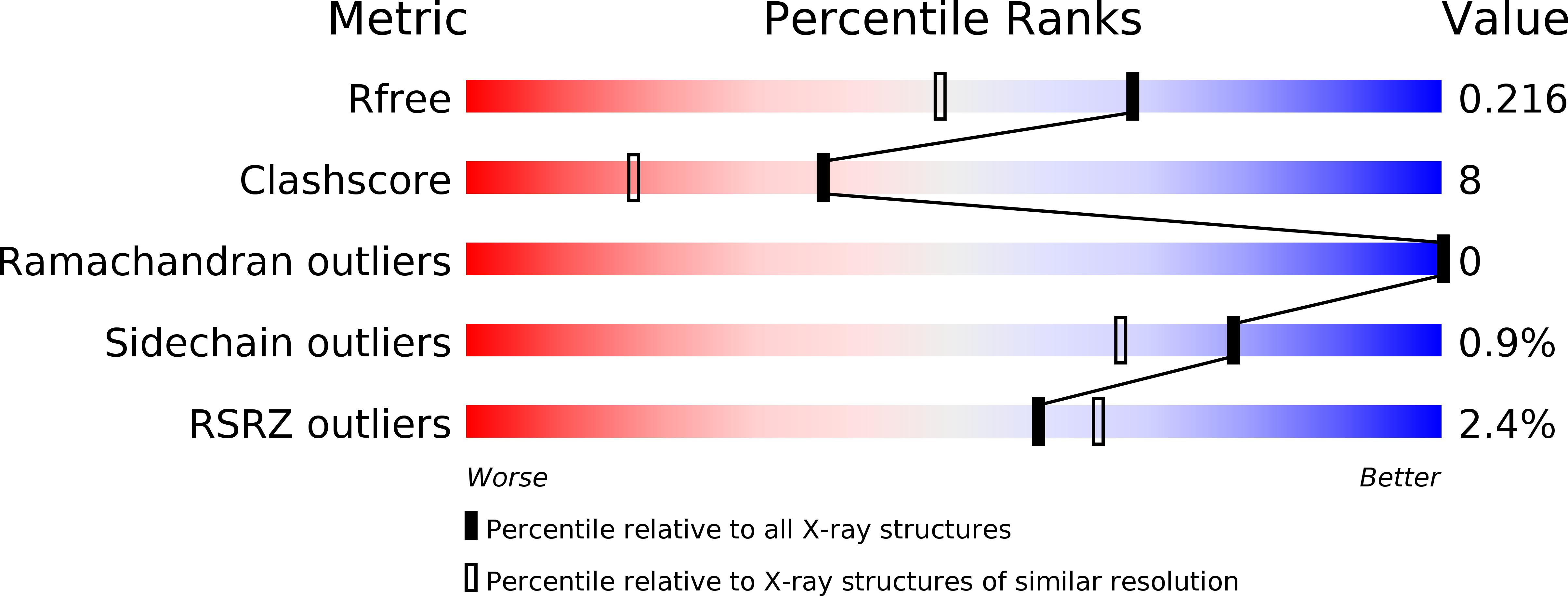
Resolution:
1.75 Å
R-Value Free:
0.21
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
P 21 21 21