Deposition Date
2014-05-07
Release Date
2014-11-05
Last Version Date
2024-11-06
Entry Detail
PDB ID:
4QBB
Keywords:
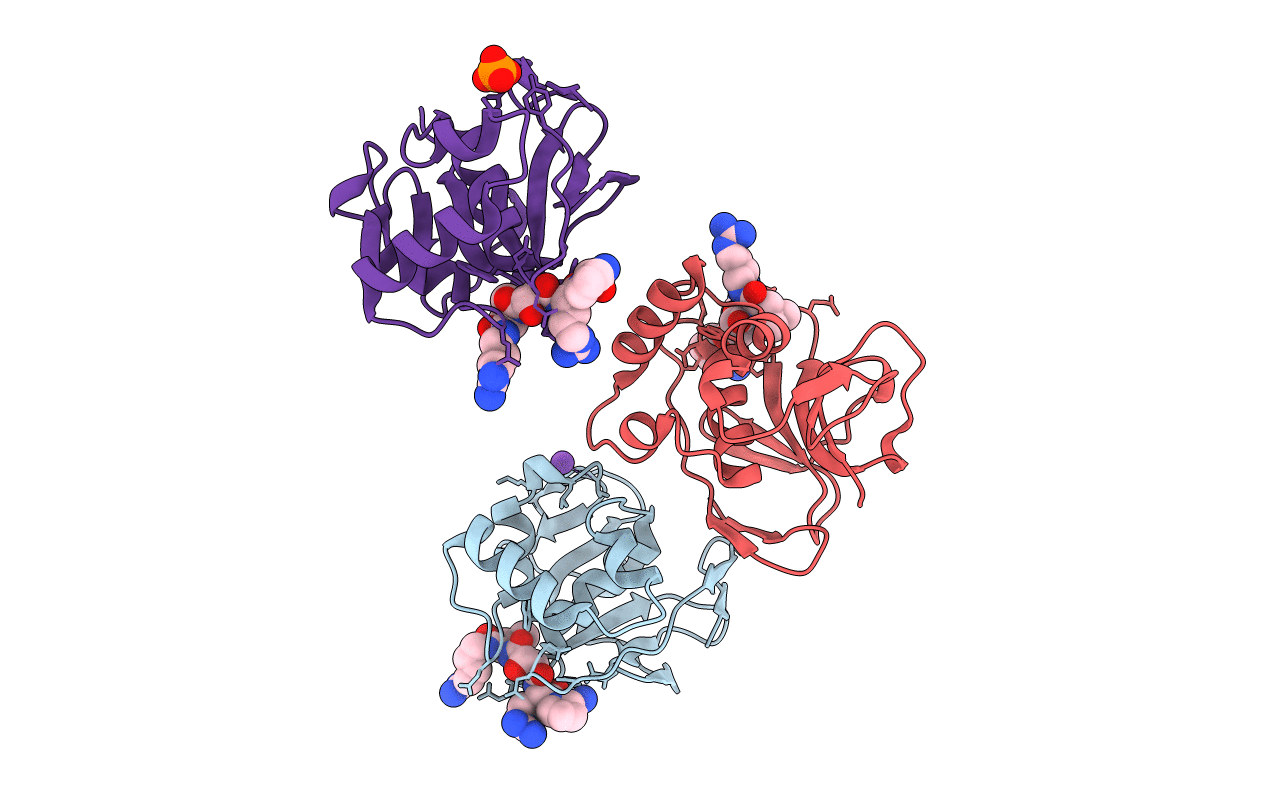
Title:
Structure of the foot-and-mouth disease virus leader proteinase in complex with inhibitor (N~2~-[(3S)-4-({(2R)-1-[(4-CARBAMIMIDAMIDOBUTYL)AMINO]-4-METHYL-1-OXOPENTAN-2-YL}AMINO)-3-HYDROXY-4-OXOBUTANOYL]-L-ARGINYL-L-PROLINAMIDE)
Biological Source:
Source Organism(s):
Foot-and-mouth disease virus (Taxon ID: 73482)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
1.60 Å
R-Value Free:
0.20
R-Value Work:
0.16
R-Value Observed:
0.17
Space Group:
P 1 21 1


