Deposition Date
2014-04-26
Release Date
2014-08-13
Last Version Date
2023-11-15
Entry Detail
PDB ID:
4Q8D
Keywords:
Title:
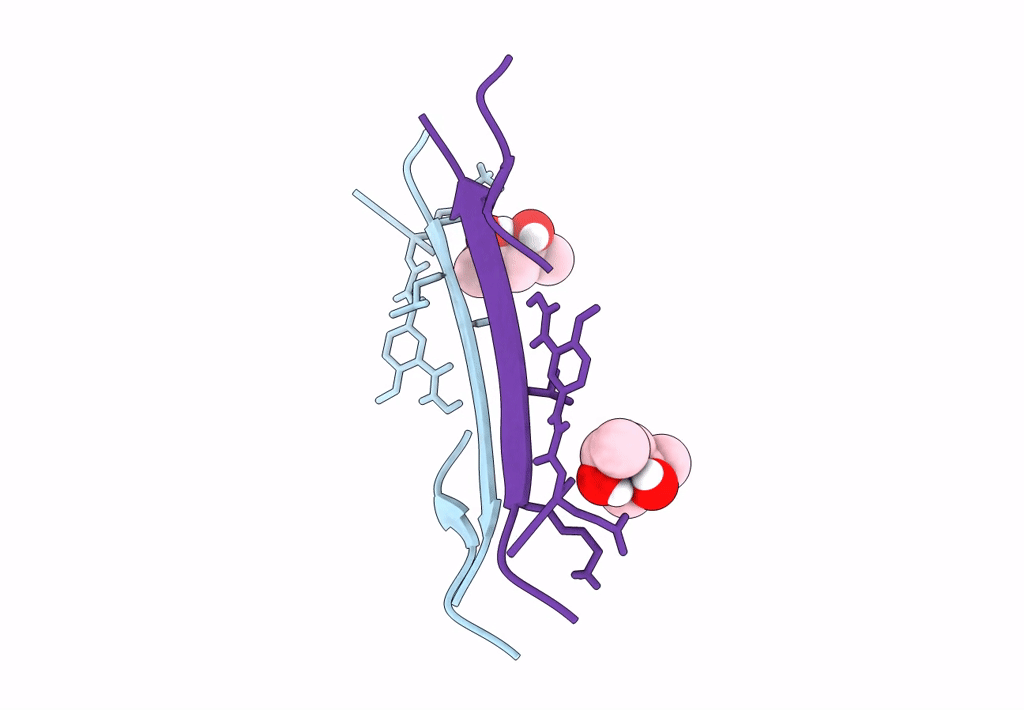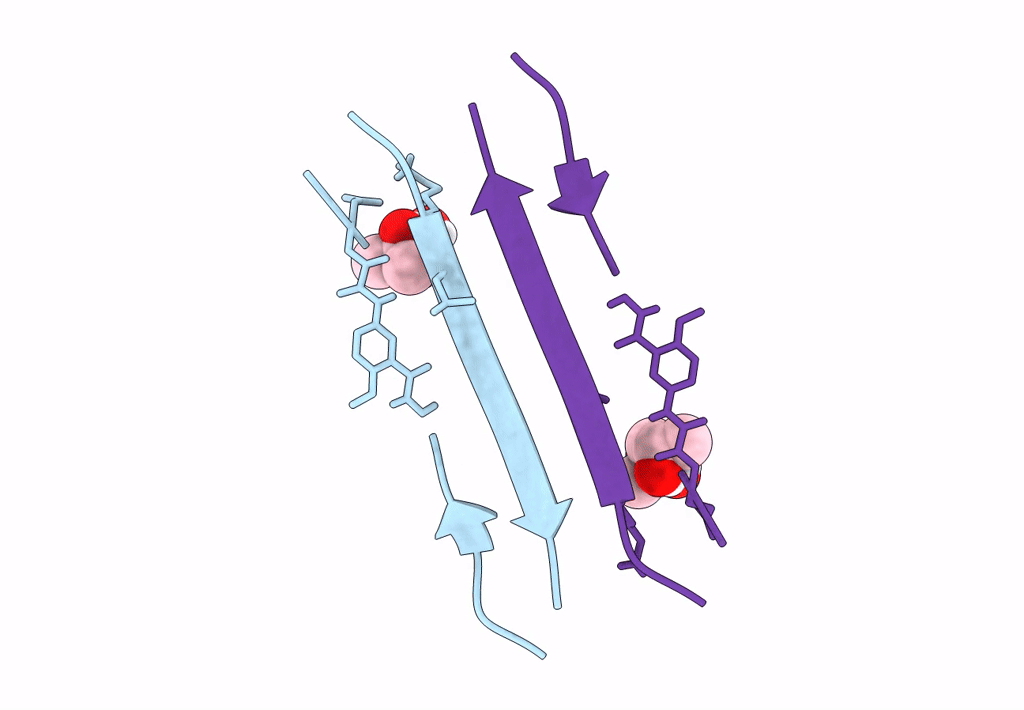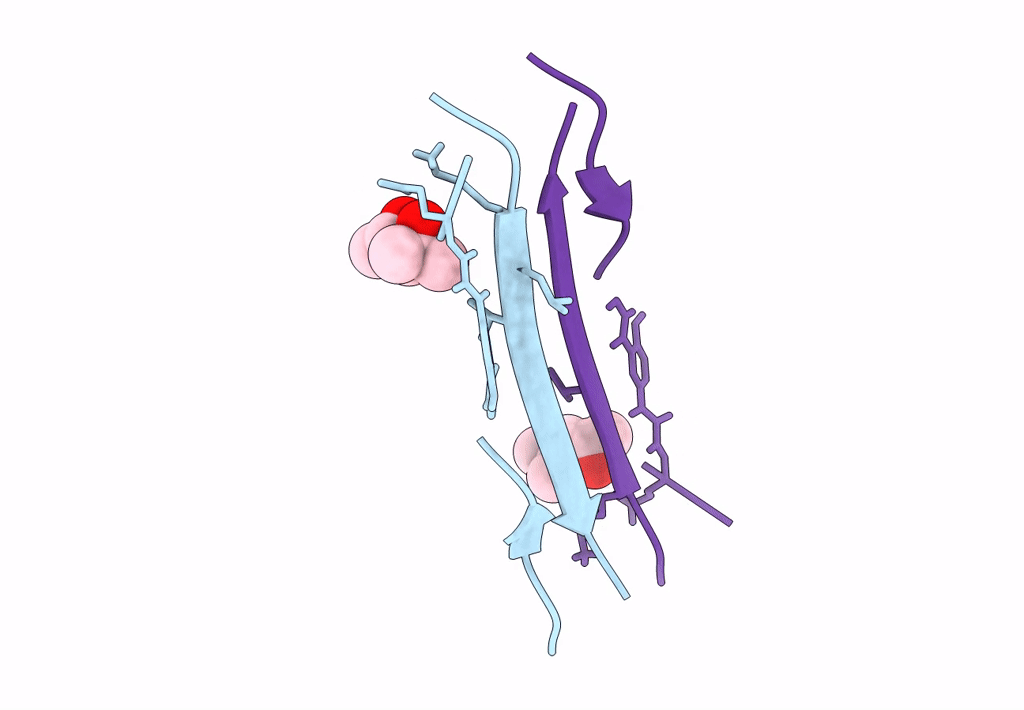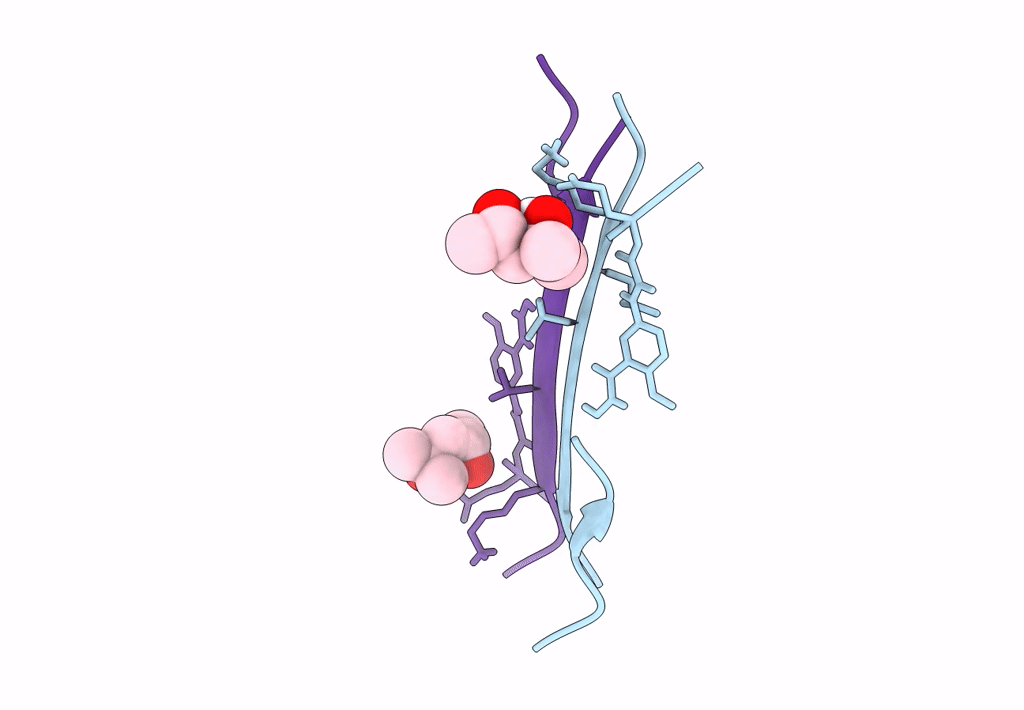
Crystal structure of a macrocyclic beta-sheet peptide containing two beta-strands from amyloid beta residues 15-23
Method Details:
Experimental Method:
Resolution:
1.75 Å
R-Value Free:
0.21
R-Value Work:
0.17
R-Value Observed:
0.18
Space Group:
C 1 2 1


