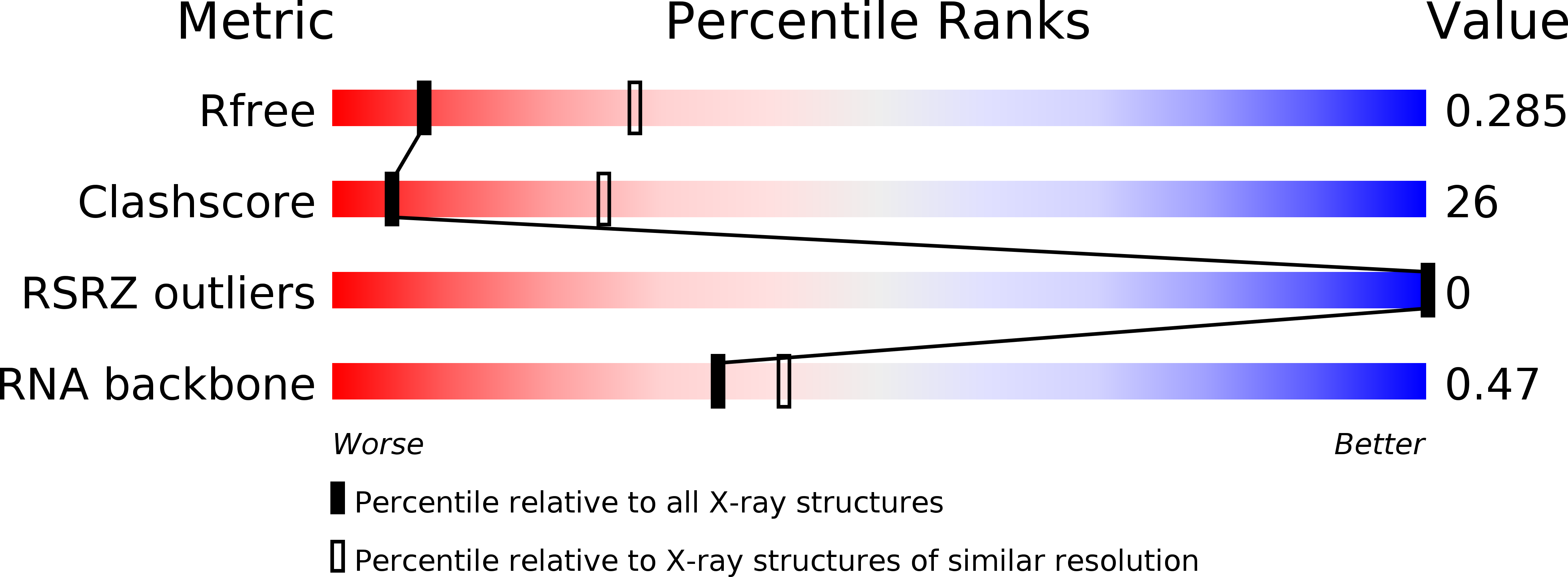Deposition Date
2014-05-07
Release Date
2015-02-18
Last Version Date
2023-12-27
Entry Detail
PDB ID:
4PHY
Keywords:
Title:
Functional conservation despite structural divergence in ligand-responsive RNA switches
Biological Source:
Source Organism(s):
Seneca valley virus (Taxon ID: 390157)
Method Details:
Experimental Method:
Resolution:
3.10 Å
R-Value Free:
0.26
R-Value Work:
0.21
R-Value Observed:
0.21
Space Group:
P 63 2 2