Deposition Date
2014-04-14
Release Date
2015-04-22
Last Version Date
2023-11-15
Entry Detail
PDB ID:
4PC8
Keywords:
Title:
Structure-based protein engineering efforts on the scaffold of a monomeric triosephosphate isomerase yielding a sugar isomerase
Biological Source:
Source Organism(s):
Trypanosoma brucei brucei (Taxon ID: 5702)
Expression System(s):
Method Details:
Experimental Method:
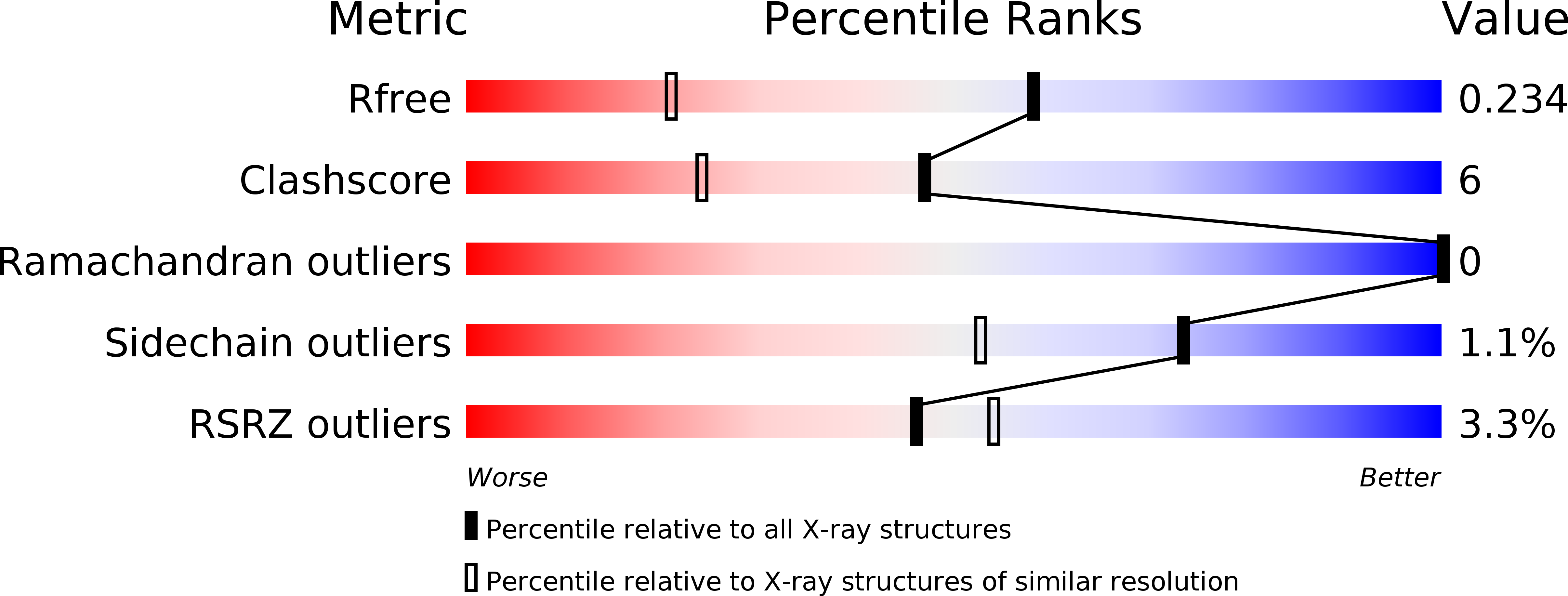
Resolution:
1.55 Å
R-Value Free:
0.21
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 43 21 2