Deposition Date
2014-03-13
Release Date
2015-05-06
Last Version Date
2023-12-27
Entry Detail
PDB ID:
4P4V
Keywords:
Title:
Hexamer formed by a macrocyclic peptide derived from beta-2-microglobulin (63-69) - (ORN)YLL(PHI)YTE(ORN)KVA(MAA)AVK
Biological Source:
Source Organism(s):
synthetic construct (Taxon ID: 32630)
Method Details:
Experimental Method:
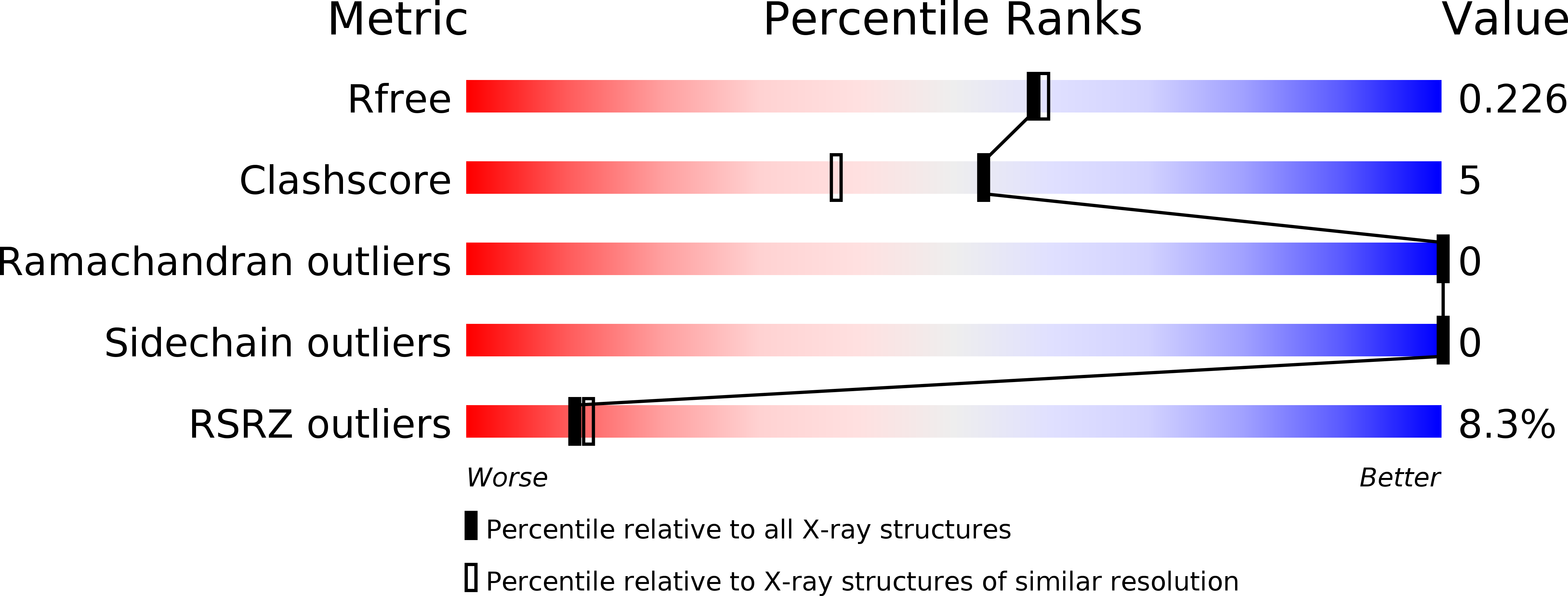
Resolution:
1.97 Å
R-Value Free:
0.22
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 42 2 2