Deposition Date
2014-02-19
Release Date
2014-07-30
Last Version Date
2023-12-27
Entry Detail
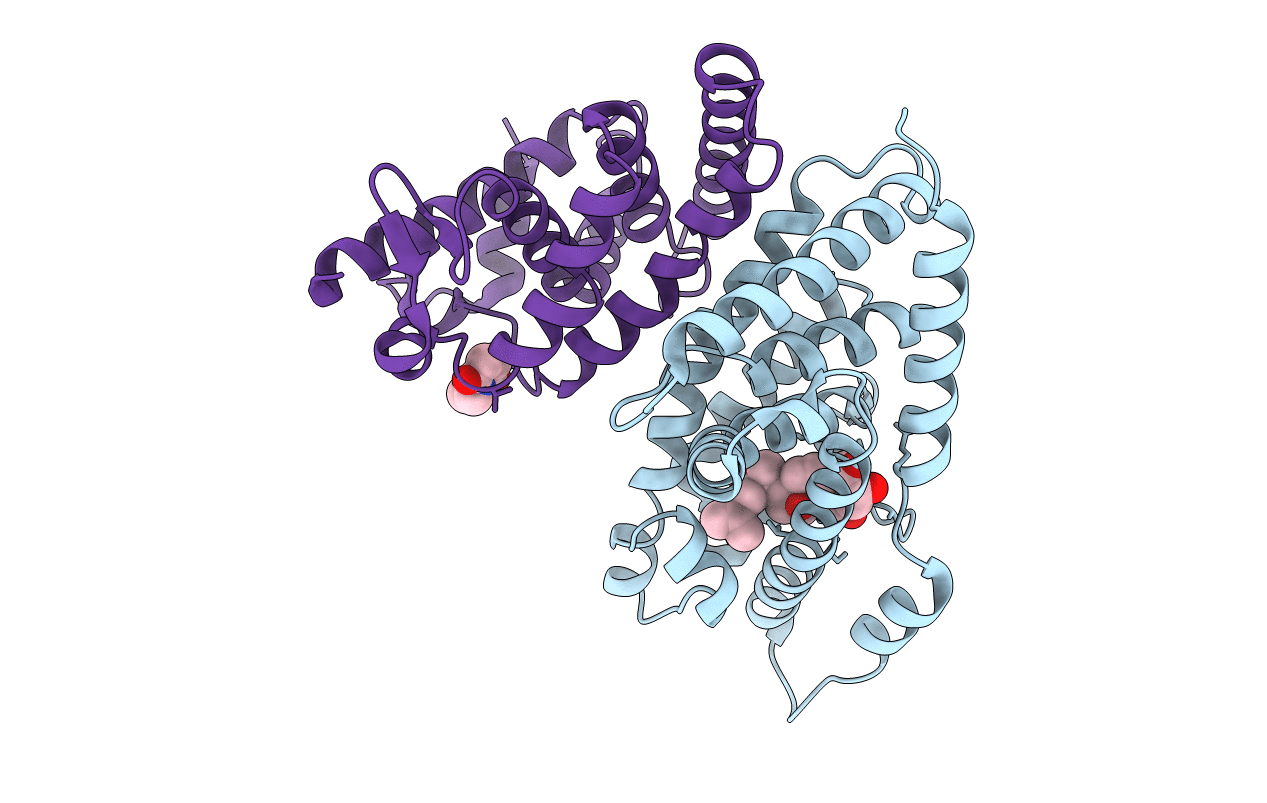
PDB ID:
4OZT
Keywords:
Title:
crystal structure of the ligand binding domains of the Bovicola ovis ecdysone receptor EcR/USP heterodimer (PonA crystal)
Biological Source:
Source Organism(s):
Pediculus humanus subsp. corporis (Taxon ID: 121224)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.70 Å
R-Value Free:
0.21
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
P 43 21 2


