Deposition Date
2014-02-11
Release Date
2014-12-03
Last Version Date
2024-02-28
Entry Detail
PDB ID:
4OR7
Keywords:
Title:
Klebsiella pneumoniae dihydrofolate reductase complexed with NADPH and 6-ethyl-5-{3-[3-(pyrimidin-5-yl)phenyl]prop-1-yn-1-yl}pyrimidine-2,4-diamine
Biological Source:
Source Organism(s):
Klebsiella pneumoniae CG43 (Taxon ID: 1244085)
Expression System(s):
Method Details:
Experimental Method:
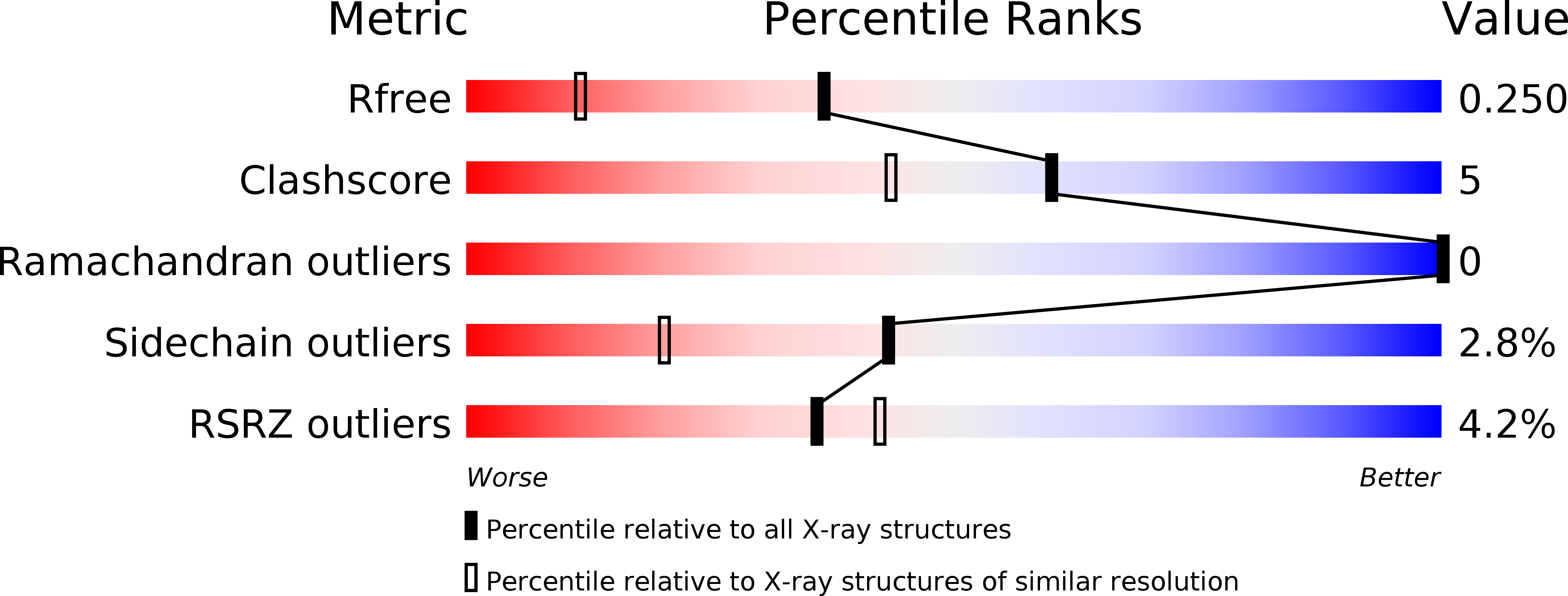
Resolution:
1.76 Å
R-Value Free:
0.25
R-Value Work:
0.21
R-Value Observed:
0.21
Space Group:
P 32 2 1