Deposition Date
2014-01-27
Release Date
2015-01-28
Last Version Date
2024-12-25
Entry Detail
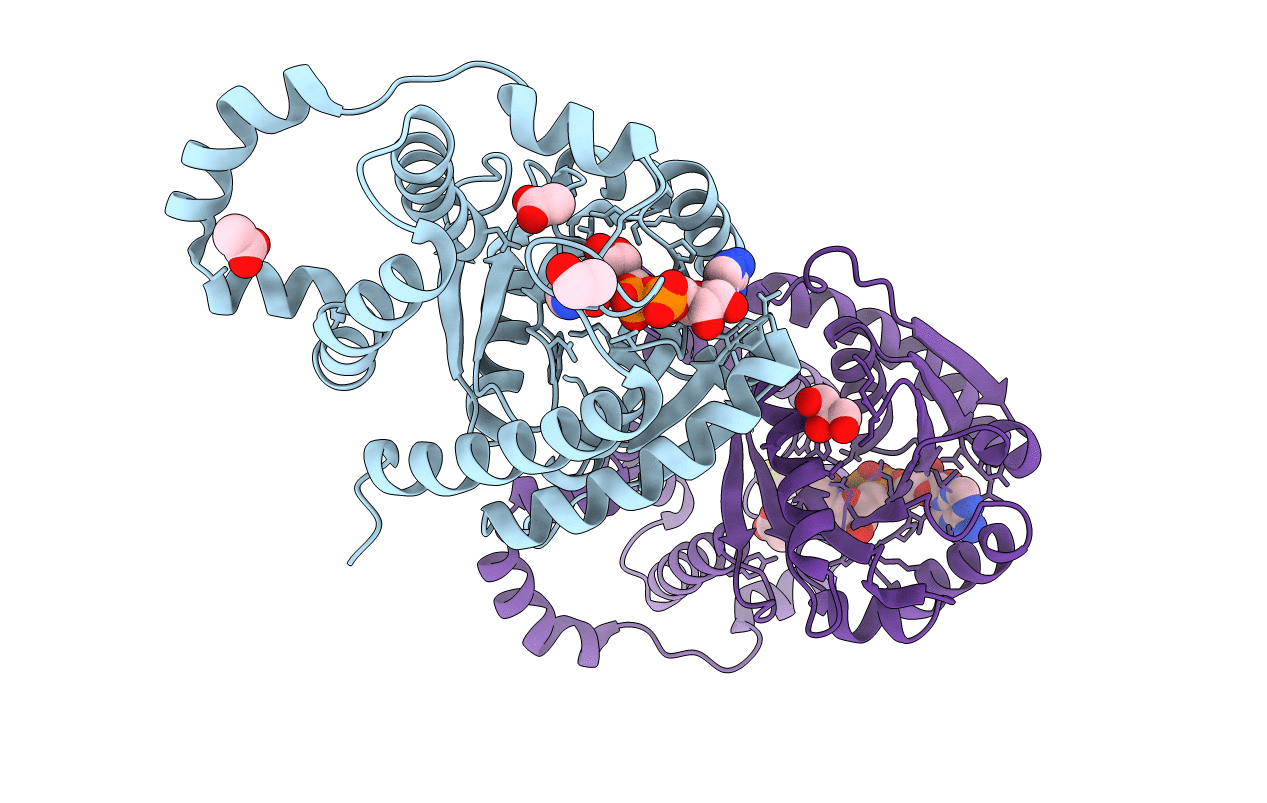
PDB ID:
4OM8
Keywords:
Title:
Crystal structure of 5-formly-3-hydroxy-2-methylpyridine 4-carboxylic acid (FHMPC) 5-dehydrogenase, an NAD+ dependent dismutase.
Biological Source:
Source Organism(s):
Mesorhizobium japonicum MAFF 303099 (Taxon ID: 266835)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
1.55 Å
R-Value Free:
0.18
R-Value Work:
0.15
R-Value Observed:
0.15
Space Group:
C 1 2 1


