Deposition Date
2014-01-07
Release Date
2014-11-26
Last Version Date
2023-09-20
Entry Detail
PDB ID:
4OBD
Keywords:
Title:
Crystal Structure of Nelfinavir-Resistant, Inactive HIV-1 Protease (D30N/N88D) in Complex with the p1-p6 substrate variant (L449F/S451N)
Biological Source:
Source Organism(s):
Human immunodeficiency virus type 1 (Taxon ID: 11685)
Human immunodeficiency virus 1 (Taxon ID: 11676)
Human immunodeficiency virus 1 (Taxon ID: 11676)
Expression System(s):
Method Details:
Experimental Method:
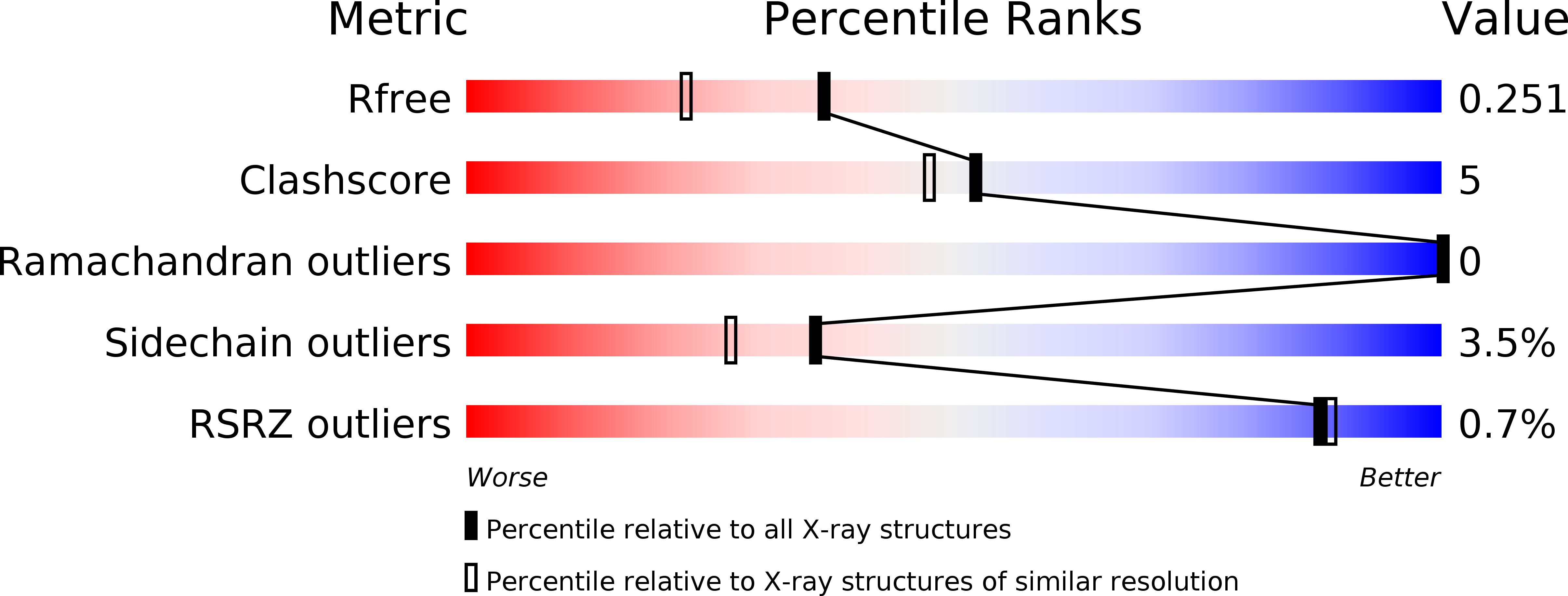
Resolution:
1.90 Å
R-Value Free:
0.24
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 1 21 1