Deposition Date
2013-12-02
Release Date
2014-10-15
Last Version Date
2024-11-06
Entry Detail
PDB ID:
4NTT
Keywords:
Title:
Structure of the catalytic subunit of cAMP-dependent protein kinase bound to ADP and one magnesium ion
Biological Source:
Source Organism(s):
Mus musculus (Taxon ID: 10090)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.50 Å
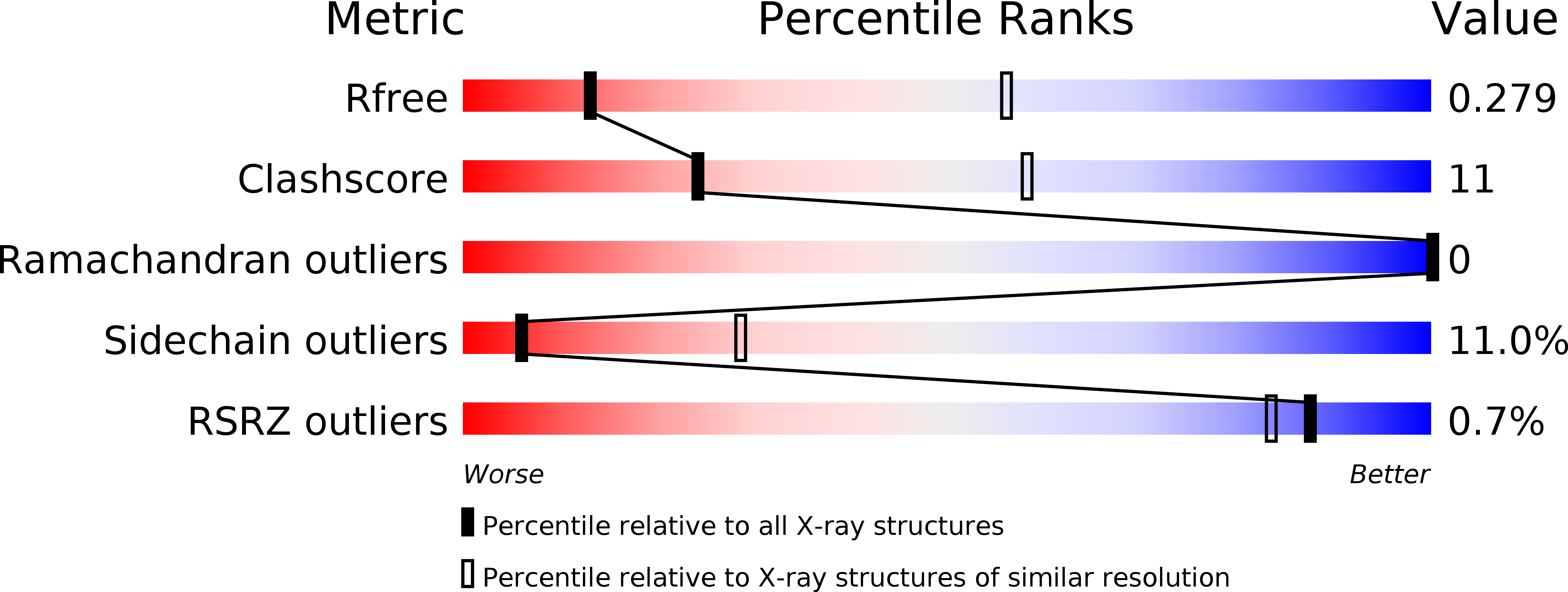
R-Value Free:
0.28
R-Value Work:
0.22
R-Value Observed:
0.23
Space Group:
P 1 21 1