Deposition Date
2013-11-14
Release Date
2014-03-19
Last Version Date
2024-04-03
Entry Detail
PDB ID:
4NLD
Keywords:
Title:
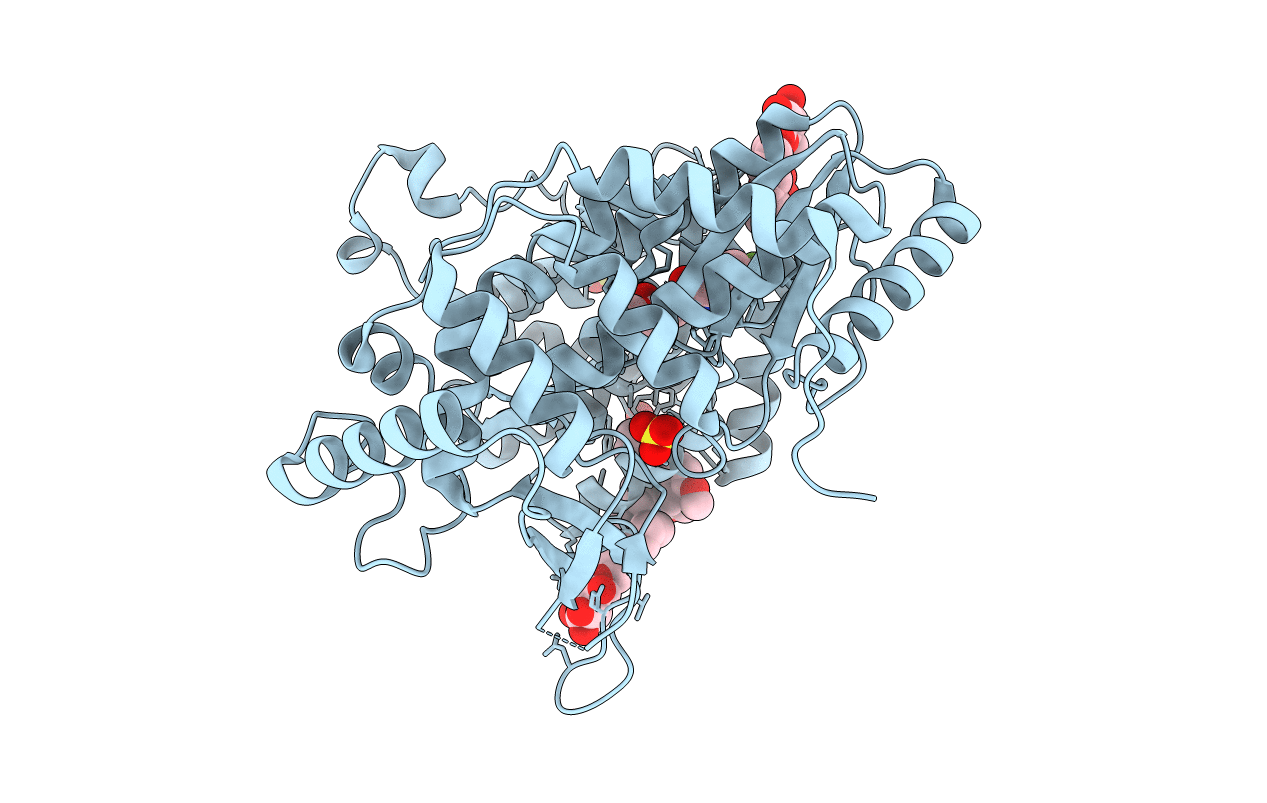
Crystal structure of the hepatitis C virus NS5B RNA-dependent RNA polymerase complex with BMS-791325 also known as (1aR,12bS)-8-cyclohexyl-n-(dimethylsulfamoyl)-11-methoxy-1a-{[(1R,5S)-3-methyl-3,8-diazabicyclo[3.2.1]oct-8-yl]carbonyl}-1,1a,2,12b-tetrahydrocyclopropa[d]indolo[2,1-a][2]benzazepine-5-carboxamide and 2-(4-fluorophenyl)-n-methyl-6-[(methylsulfonyl)amino]-5-(propan-2-yloxy)-1-benzofuran-3-carboxamide
Biological Source:
Source Organism(s):
Hepatitis C virus (Taxon ID: 333284)
Expression System(s):
Method Details:
Experimental Method:
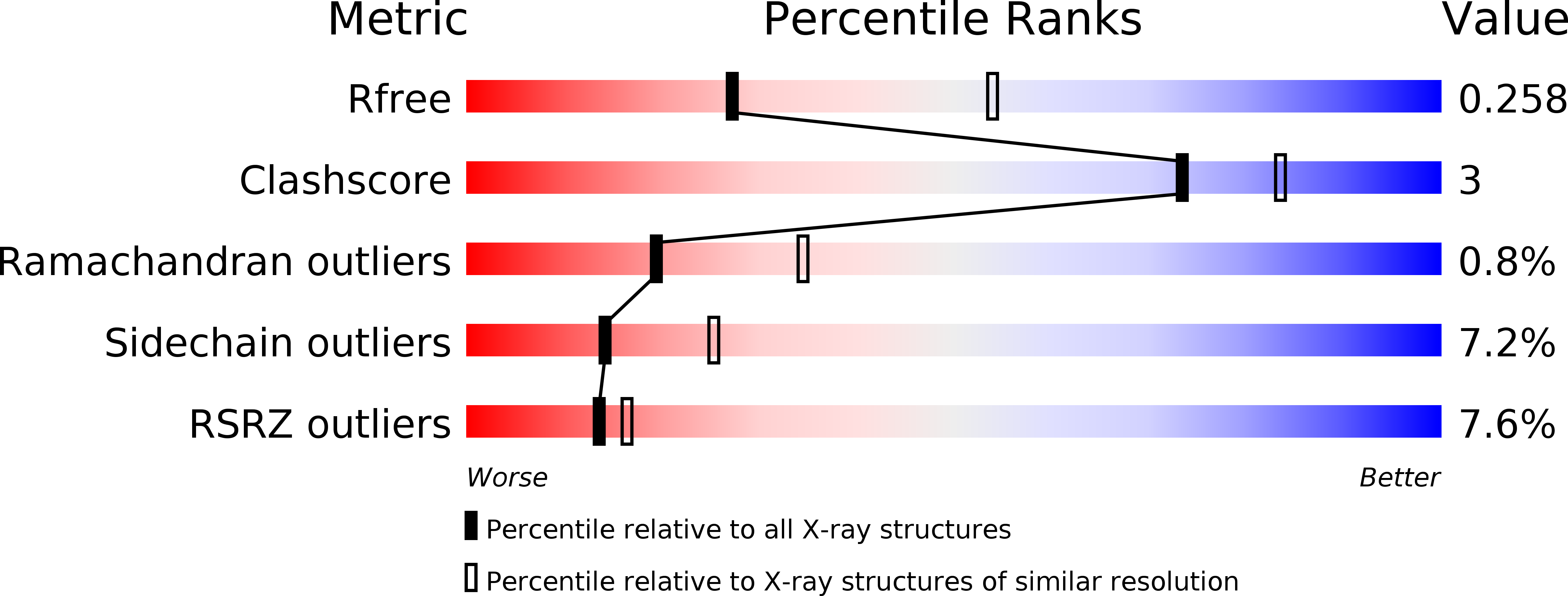
Resolution:
2.75 Å
R-Value Free:
0.25
R-Value Work:
0.21
R-Value Observed:
0.21
Space Group:
I 2 2 2