Deposition Date
2013-11-01
Release Date
2014-05-14
Last Version Date
2024-11-13
Entry Detail
PDB ID:
4NFW
Keywords:
Title:
Structure and atypical hydrolysis mechanism of the Nudix hydrolase Orf153 (YmfB) from Escherichia coli
Biological Source:
Source Organism(s):
Escherichia coli (Taxon ID: 885275)
Expression System(s):
Method Details:
Experimental Method:
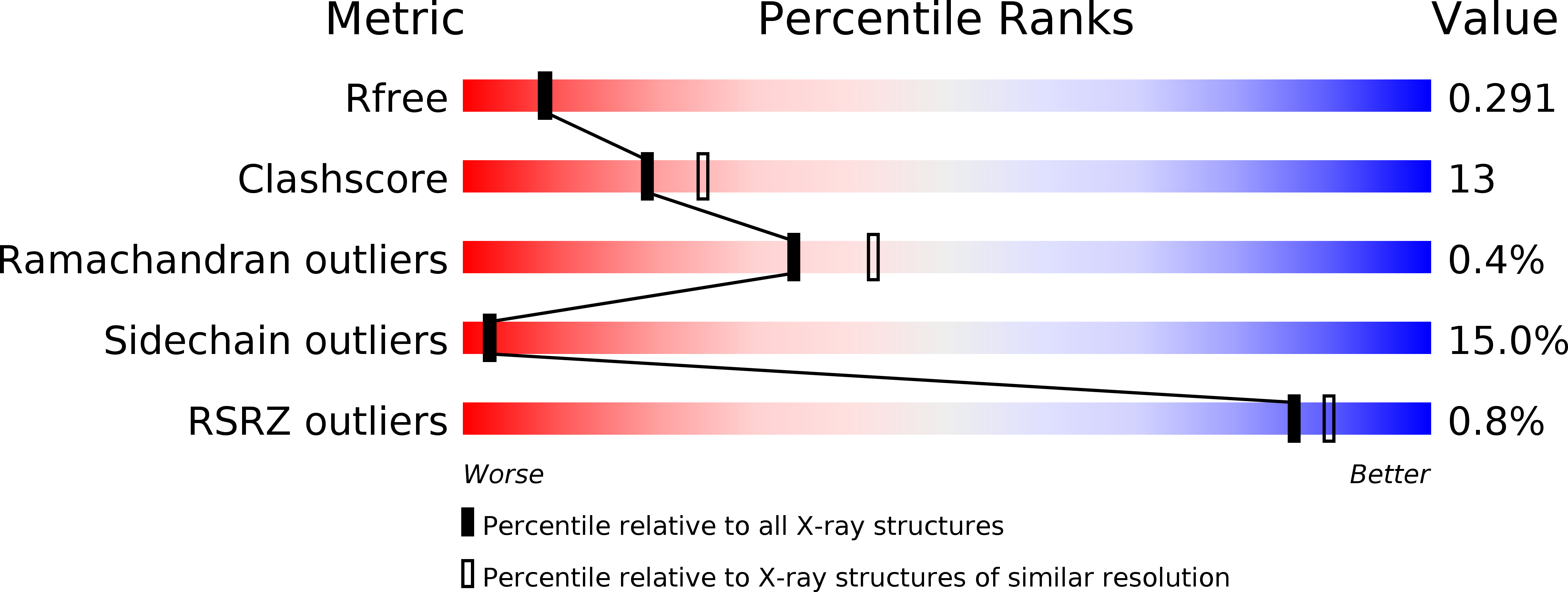
Resolution:
2.30 Å
R-Value Free:
0.28
R-Value Work:
0.24
R-Value Observed:
0.24
Space Group:
P 41