Deposition Date
2013-10-04
Release Date
2014-02-12
Last Version Date
2024-02-28
Entry Detail
PDB ID:
4N1Y
Keywords:
Title:
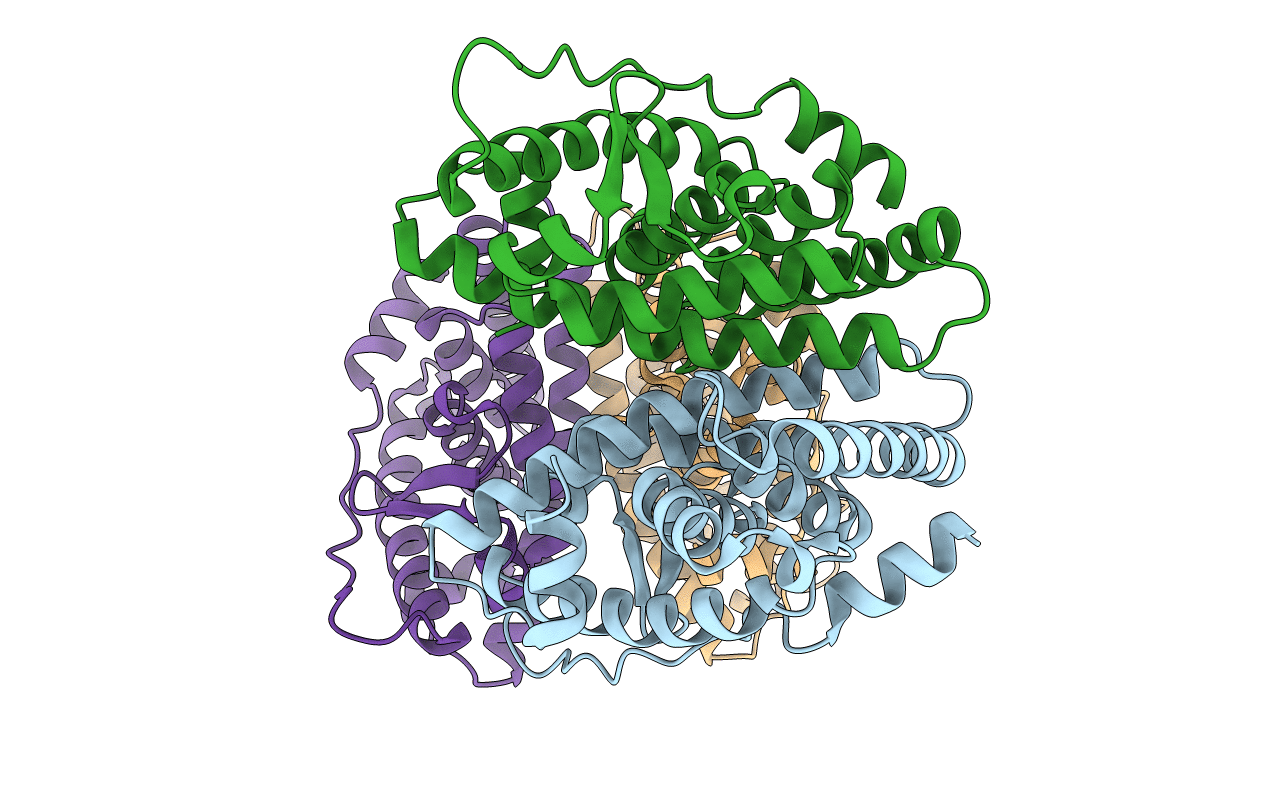
Crystal Structure of the Pacific Oyster Estrogen Receptor Ligand Binding Domain
Biological Source:
Source Organism:
Crassostrea gigas (Taxon ID: 29159)
Host Organism:
Method Details:
Experimental Method:
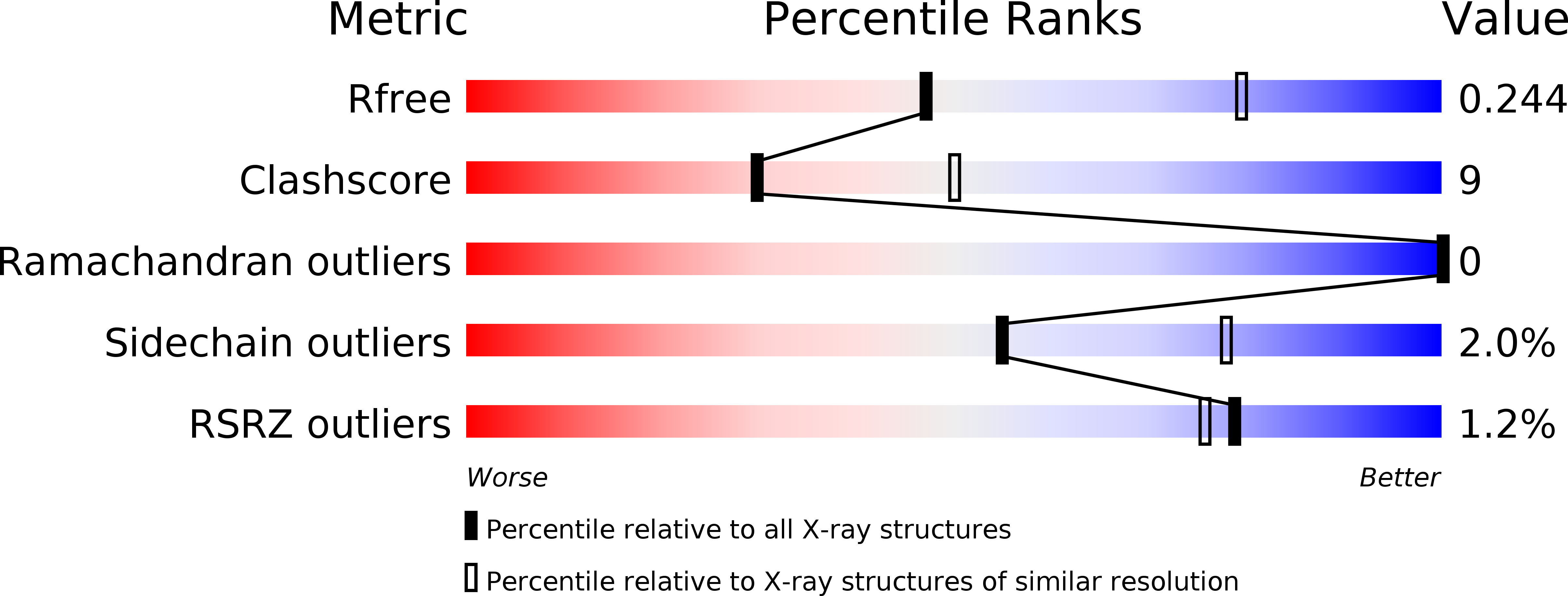
Resolution:
2.61 Å
R-Value Free:
0.24
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 21 21 21