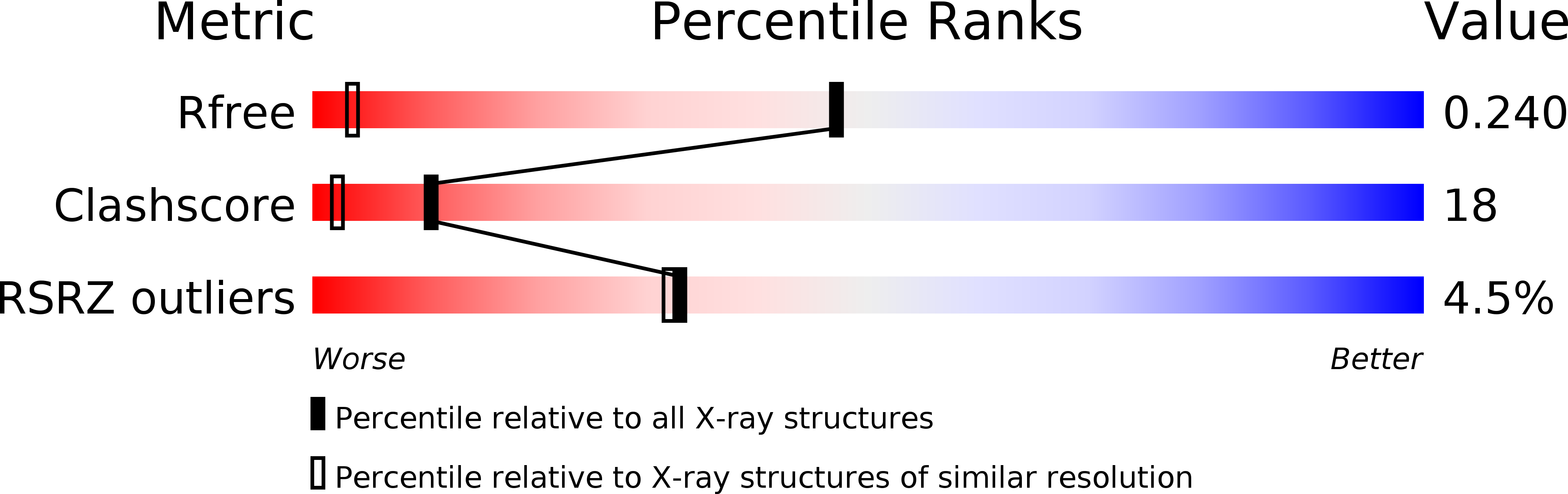Deposition Date
2013-09-05
Release Date
2013-11-06
Last Version Date
2024-02-28
Entry Detail
PDB ID:
4MKW
Keywords:
Title:
Comparison of the structural and dynamic effects of 5-methylcytosine and 5-chlorocytosine in a CpG dinucleotide sequence
Method Details:
Experimental Method:
Resolution:
1.22 Å
R-Value Free:
0.24
R-Value Work:
0.21
R-Value Observed:
0.21
Space Group:
P 21 21 21