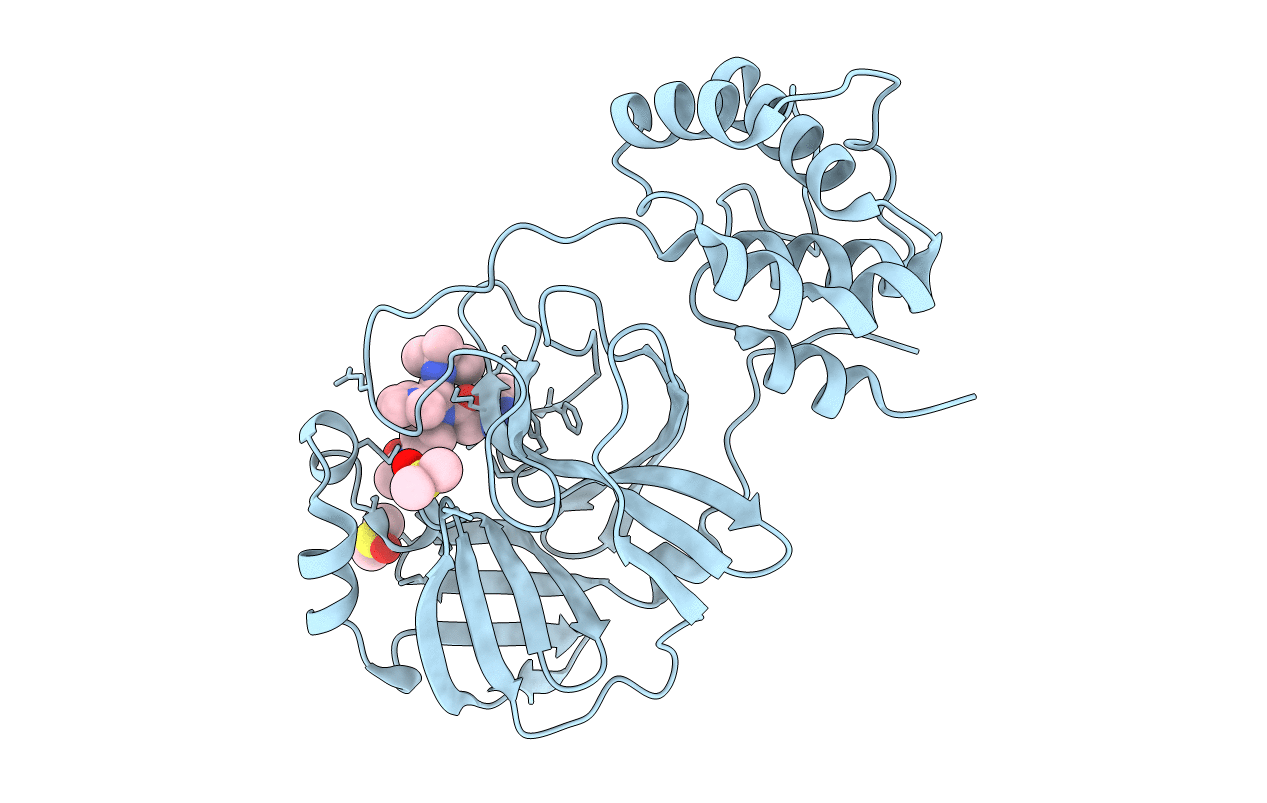
Deposition Date
2013-08-23
Release Date
2013-10-02
Last Version Date
2024-02-28
Entry Detail
PDB ID:
4MDS
Keywords:
Title:
Discovery of N-(benzo[1,2,3]triazol-1-yl)-N-(benzyl)acetamido)phenyl) carboxamides as severe acute respiratory syndrome coronavirus (SARS-CoV) 3CLpro inhibitors: identification of ML300 and non-covalent nanomolar inhibitors with an induced-fit binding
Biological Source:
Source Organism(s):
SARS coronavirus (Taxon ID: 227859)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
1.60 Å
R-Value Free:
0.21
R-Value Work:
0.16
R-Value Observed:
0.17
Space Group:
C 1 2 1


