Deposition Date
2013-08-21
Release Date
2013-09-04
Last Version Date
2024-02-28
Entry Detail
PDB ID:
4MCB
Keywords:
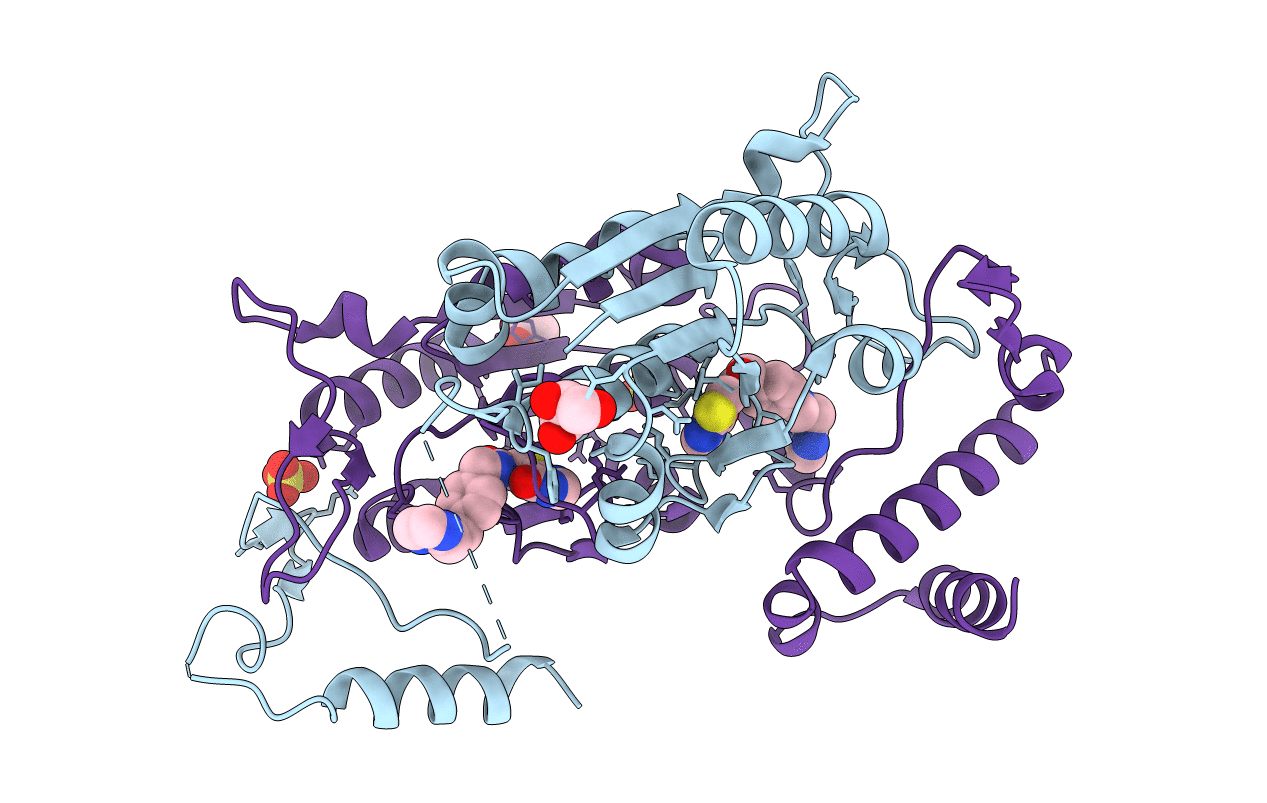
Title:
H.influenzae TrmD in complex with N-(4-{[(1H-IMIDAZOL-2-YLMETHYL)AMINO]METHYL}BENZYL)-4-OXO-3,4-DIHYDROTHIENO[2,3-D]PYRIMIDINE-5-CARBOXAMIDE
Biological Source:
Source Organism(s):
Haemophilus influenzae (Taxon ID: 71421)
Expression System(s):
Method Details:
Experimental Method:
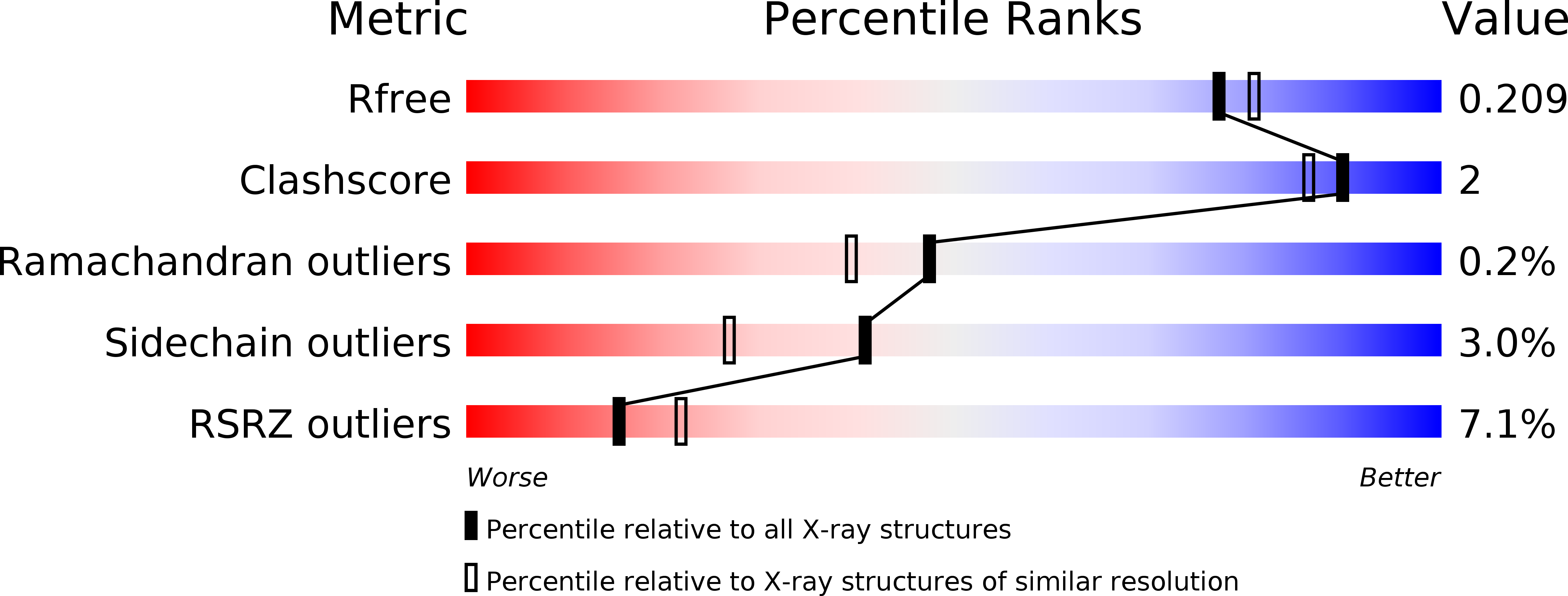
Resolution:
1.94 Å
R-Value Free:
0.20
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 41