Deposition Date
2013-07-26
Release Date
2014-09-24
Last Version Date
2024-02-28
Entry Detail
PDB ID:
4LVL
Keywords:
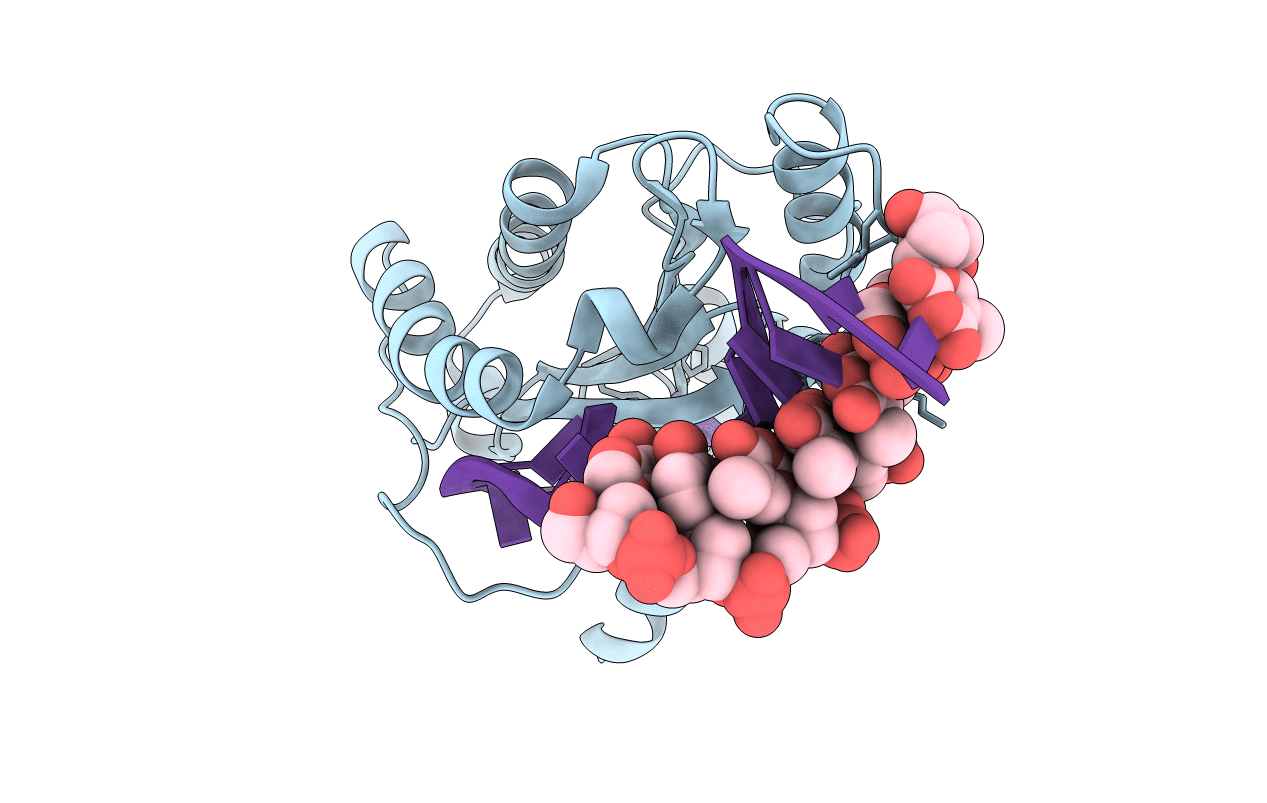
Title:
MobM Relaxase Domain (MOBV; Mob_Pre) bound to plasmid pMV158 oriT DNA (22nt+3'Thiophosphate). Mn-bound crystal structure at pH 6.8
Biological Source:
Source Organism:
Streptococcus agalactiae (Taxon ID: 1311)
Synthetic DNA (Taxon ID: 32630)
Synthetic DNA (Taxon ID: 32630)
Host Organism:
Method Details:
Experimental Method:
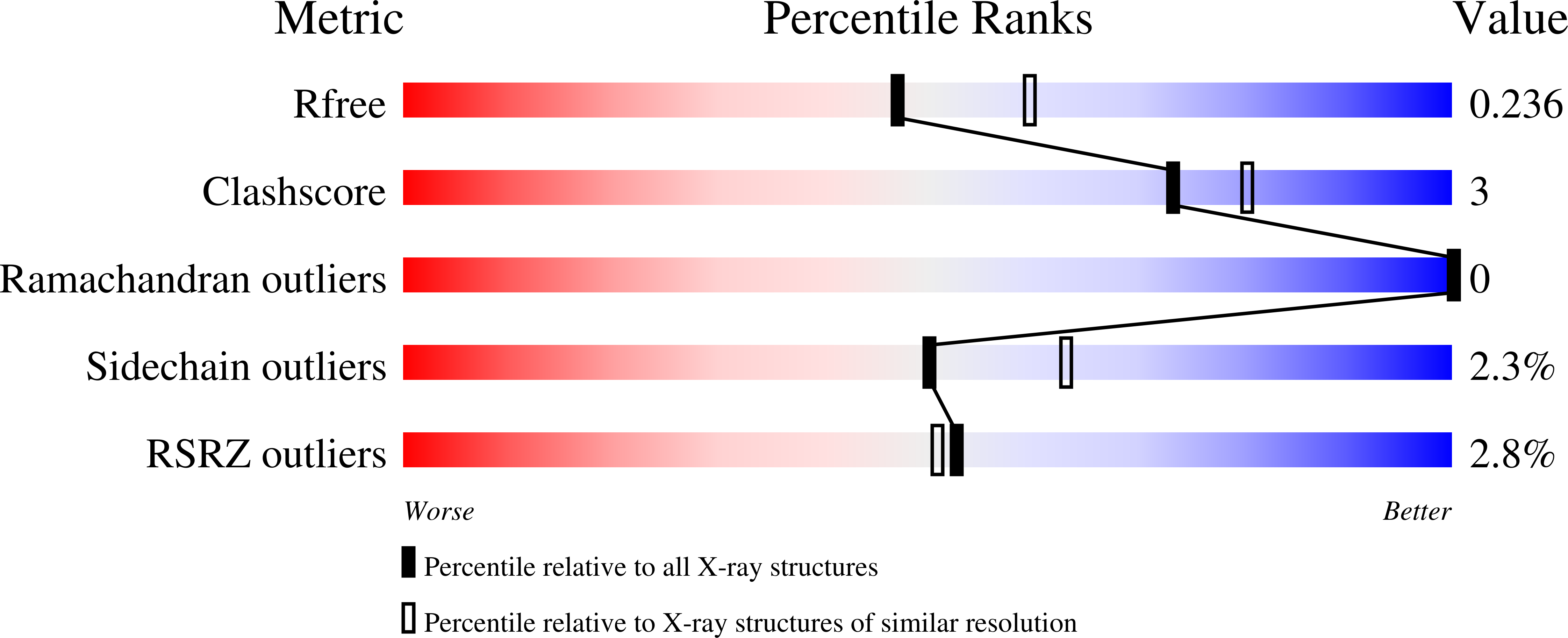
Resolution:
2.20 Å
R-Value Free:
0.23
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
P 61 2 2