Deposition Date
2013-07-22
Release Date
2013-12-25
Last Version Date
2023-09-20
Entry Detail
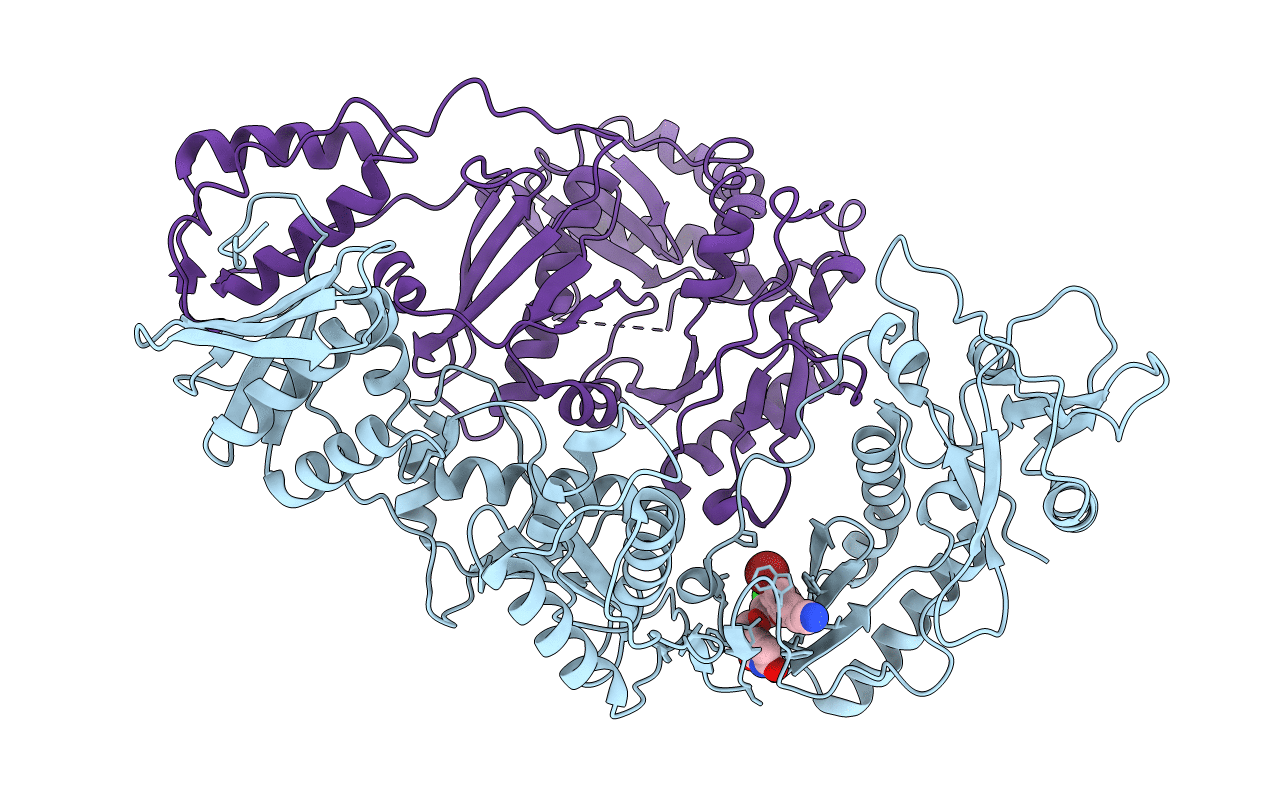
PDB ID:
4LSN
Keywords:
Title:
Crystal Structure of HIV-1 Reverse Transcriptase in Complex with (E)-3-(3-bromo-5-(4-chloro-2-(2-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)ethoxy)phenoxy)phenyl)acrylonitrile (JLJ518), a non-nucleoside inhibitor
Biological Source:
Source Organism(s):
Human immunodeficiency virus type 1 (Taxon ID: 11678)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.10 Å
R-Value Free:
0.30
R-Value Work:
0.25
R-Value Observed:
0.25
Space Group:
C 1 2 1


