Deposition Date
2013-07-20
Release Date
2013-09-04
Last Version Date
2023-11-15
Entry Detail
PDB ID:
4LRS
Keywords:
Title:
Crystal and solution structures of the bifunctional enzyme (Aldolase/Aldehyde dehydrogenase) from Thermomonospora curvata, reveal a cofactor-binding domain motion during NAD+ and CoA accommodation whithin the shared cofactor-binding site
Biological Source:
Source Organism(s):
Thermomonospora curvata (Taxon ID: 471852)
Thermomonospora curvata (Taxon ID: 2020)
Thermomonospora curvata (Taxon ID: 2020)
Expression System(s):
Method Details:
Experimental Method:
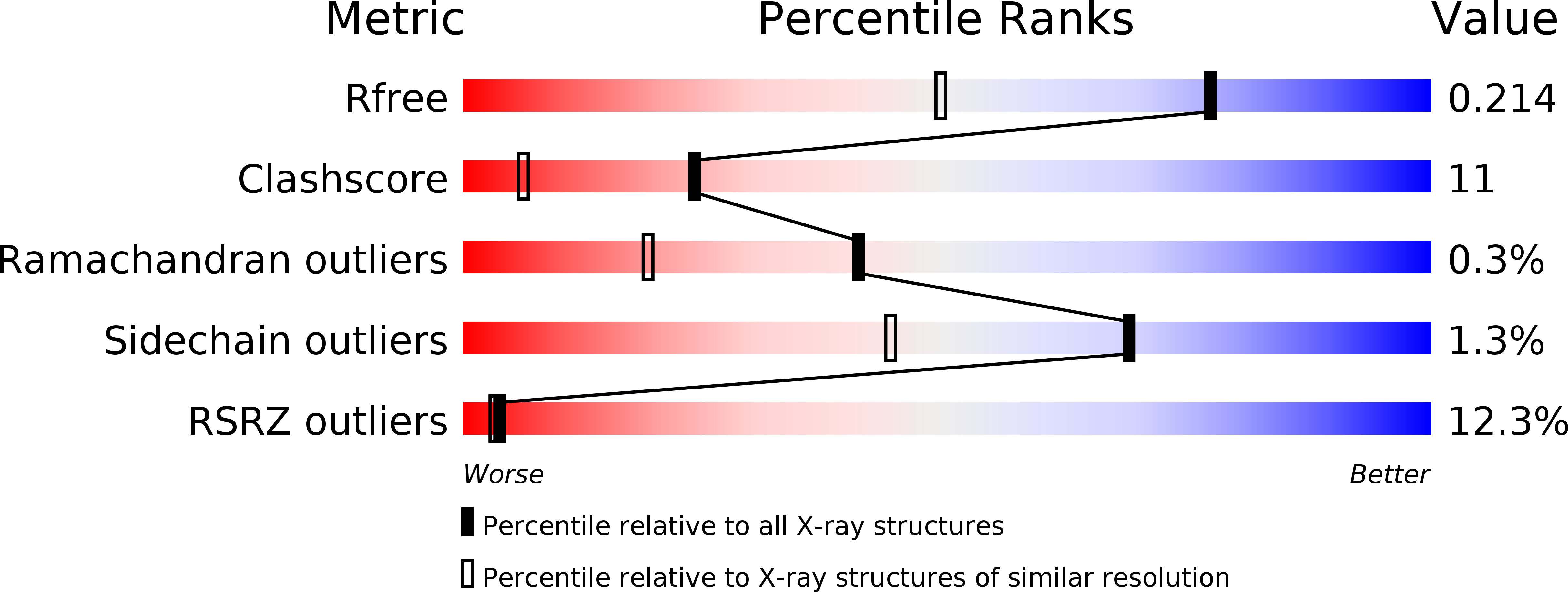
Resolution:
1.55 Å
R-Value Free:
0.20
R-Value Work:
0.16
R-Value Observed:
0.16
Space Group:
C 1 2 1