Deposition Date
2013-05-23
Release Date
2013-08-21
Last Version Date
2025-03-26
Entry Detail
PDB ID:
4KVU
Keywords:
Title:
Crystal structure of a 6-helix coiled coil CC-Hex-L17C-W224BF
Method Details:
Experimental Method:
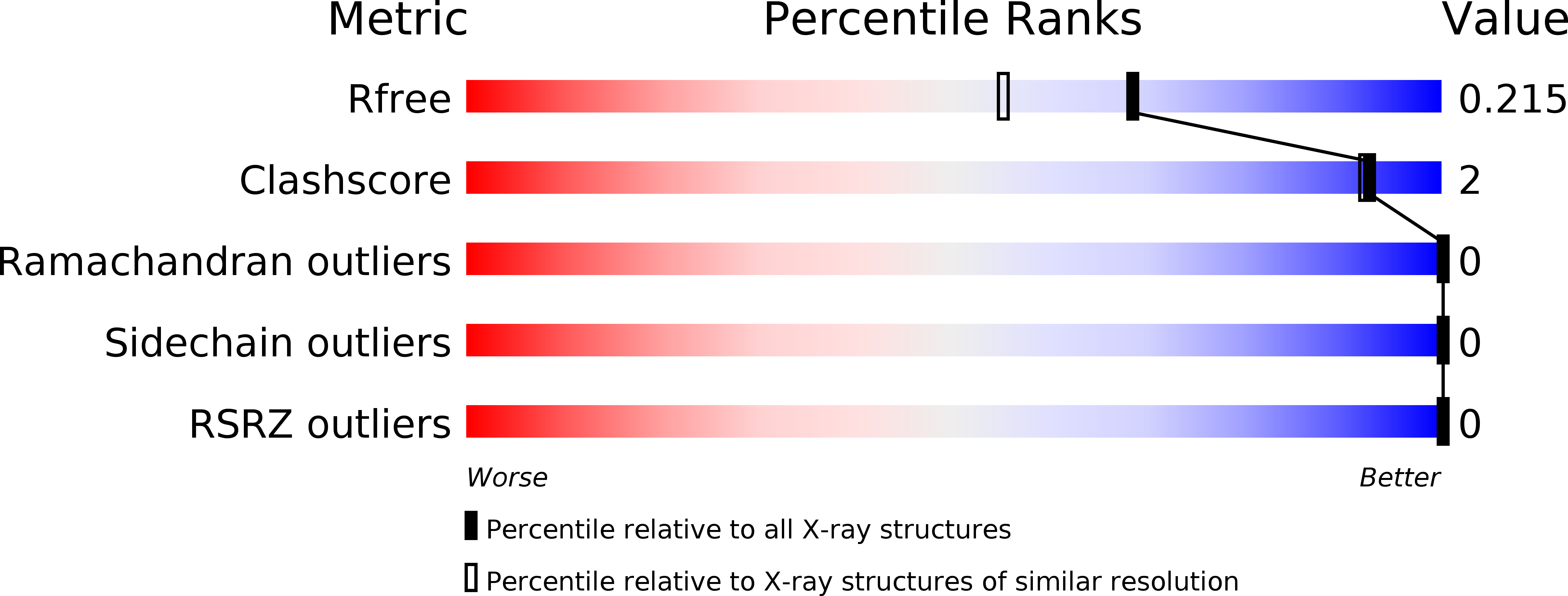
Resolution:
1.80 Å
R-Value Free:
0.21
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
F 2 2 2