Deposition Date
2013-05-03
Release Date
2013-09-04
Last Version Date
2023-09-20
Entry Detail
PDB ID:
4KJI
Keywords:
Title:
Novel re-arrangement of an RsmA/cSRa family protein to create a structurally distinct new RNA-binding family member
Biological Source:
Source Organism(s):
Pseudomonas aeruginosa (Taxon ID: 208963)
Expression System(s):
Method Details:
Experimental Method:
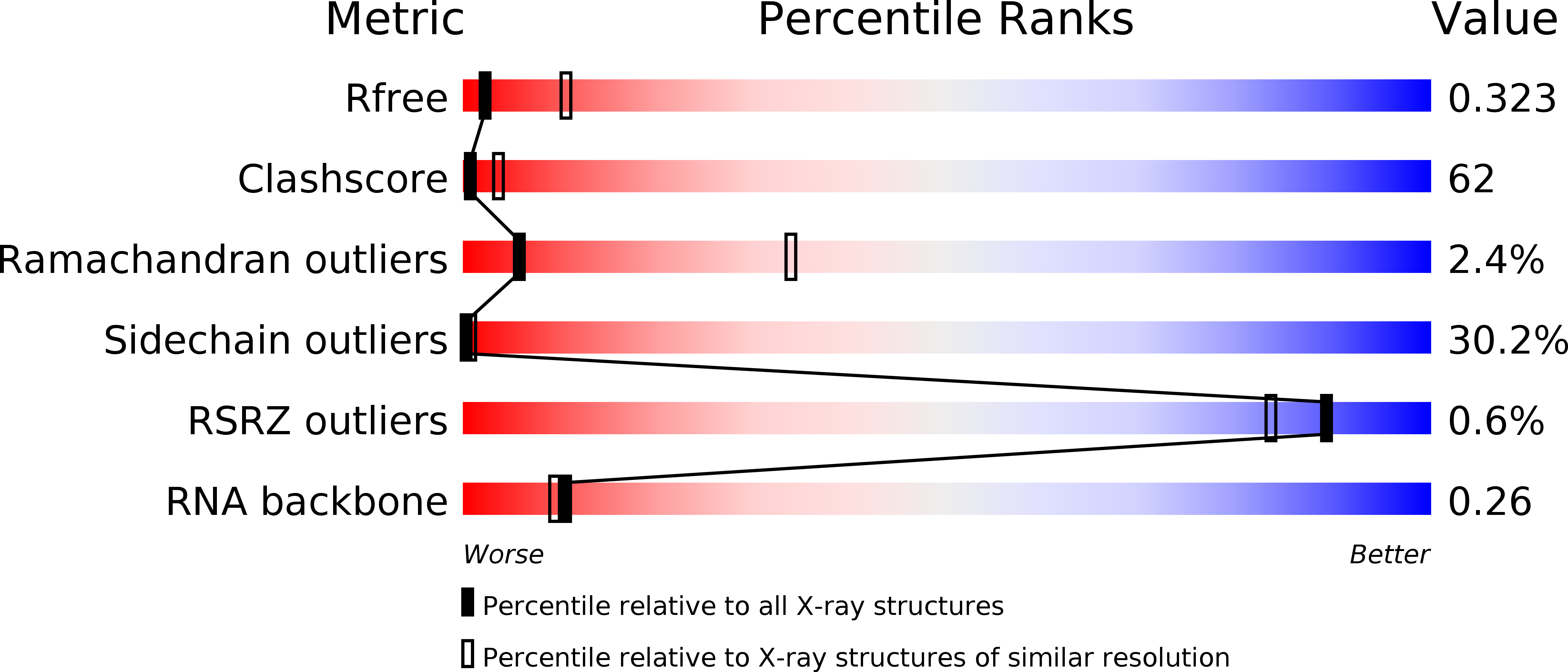
Resolution:
3.20 Å
R-Value Free:
0.31
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
P 43 2 2