Deposition Date
2013-04-11
Release Date
2014-10-01
Last Version Date
2024-02-28
Entry Detail
PDB ID:
4K43
Keywords:
Title:
Crystal structure of actin in complex with synthetic AplC tail analogue GC04 [N-{(1E,4R,5R,7E,9S,10S,11S)-4,10-dimethoxy-11-[(2S,4S,5S)-2-(4-methoxyphenyl)-5-methyl-1,3-dioxan-4-yl]-5,9-dimethyl-6-oxododeca-1,7-dien-1-yl}-N-methylformamide]
Biological Source:
Source Organism(s):
Oryctolagus cuniculus (Taxon ID: 9986)
Method Details:
Experimental Method:
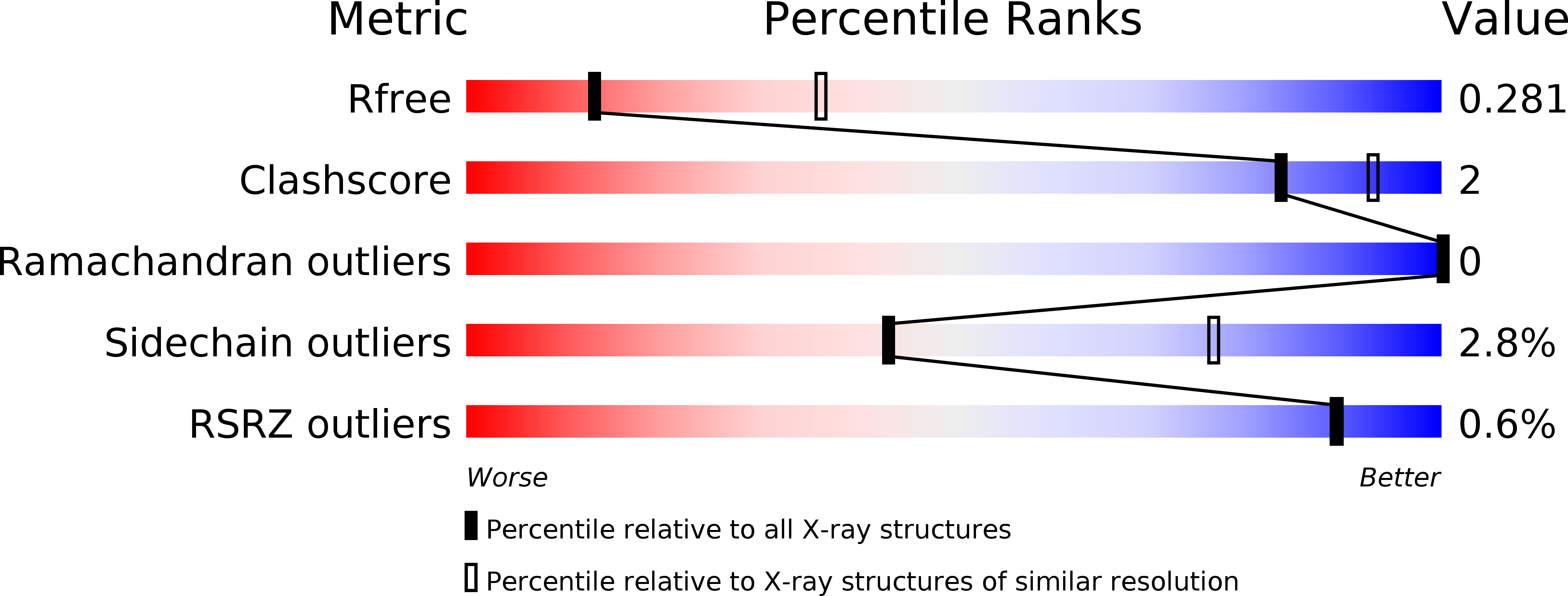
Resolution:
2.90 Å
R-Value Free:
0.27
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
P 1 21 1