Deposition Date
2013-03-28
Release Date
2013-05-01
Last Version Date
2024-02-28
Entry Detail
PDB ID:
4JXZ
Keywords:
Title:
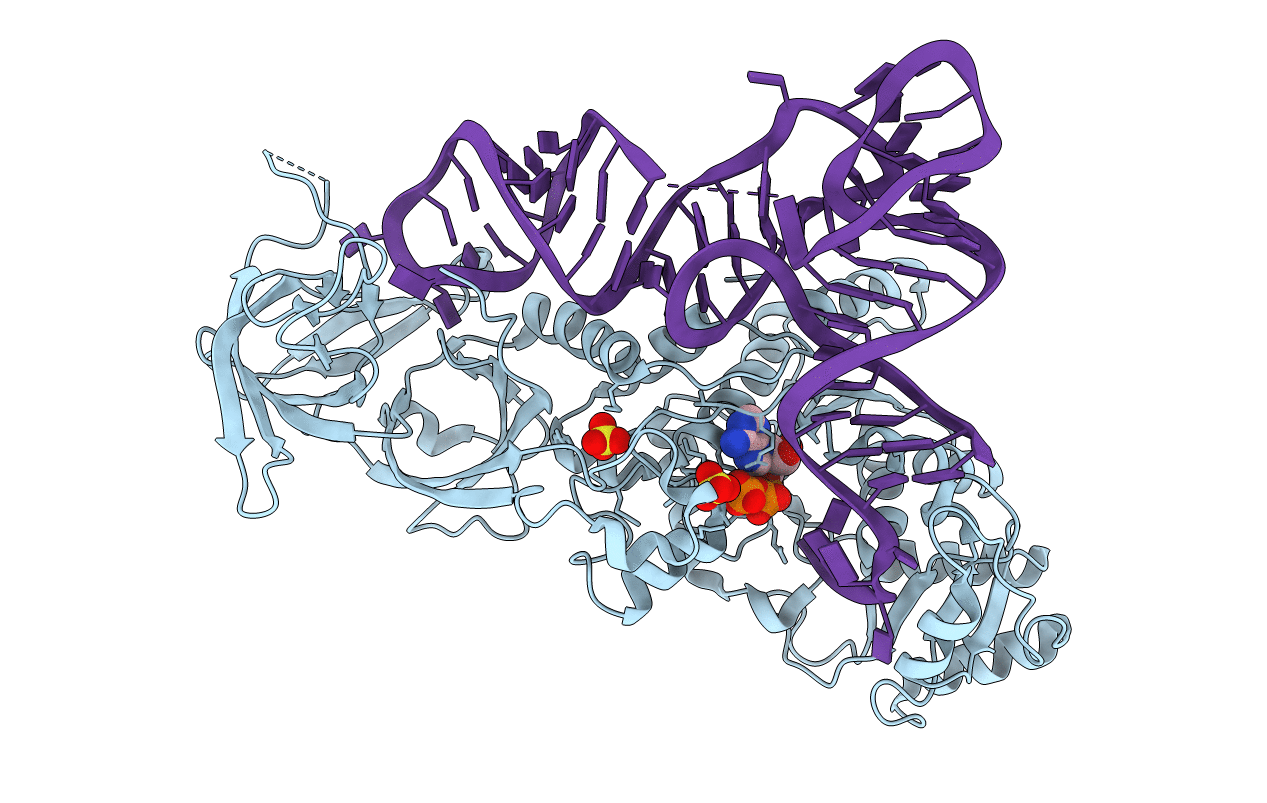
Structure of E. coli glutaminyl-tRNA synthetase bound to ATP and a tRNA(Gln) acceptor containing a UUG anticodon
Biological Source:
Source Organism(s):
Escherichia coli (Taxon ID: 83333)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
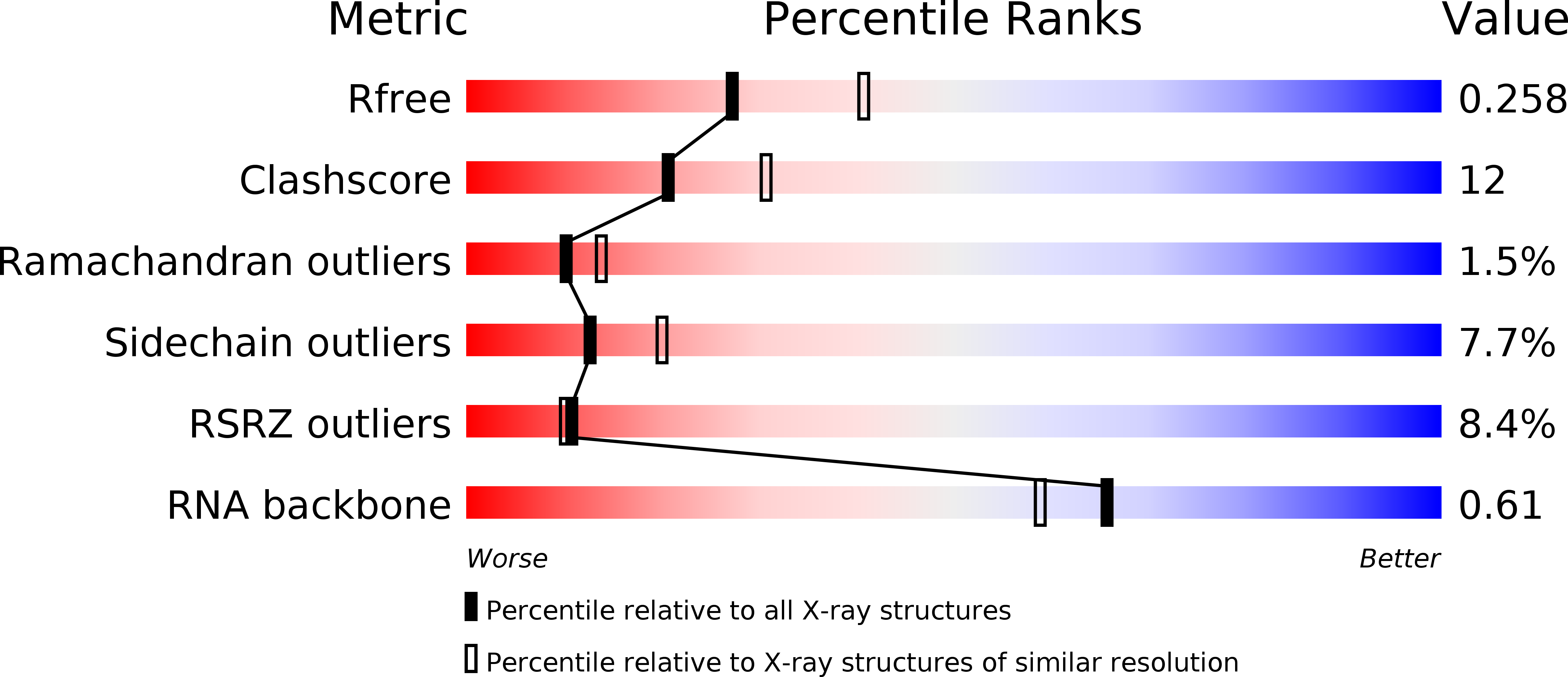
Resolution:
2.40 Å
R-Value Free:
0.26
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
C 2 2 21