Deposition Date
2013-03-22
Release Date
2013-05-01
Last Version Date
2023-11-15
Entry Detail
PDB ID:
4JSQ
Keywords:
Title:
Yeast 20S proteasome in complex with the dimerized linear mimetic of TMC-95A - yCP:4e
Biological Source:
Source Organism(s):
Saccharomyces cerevisiae (Taxon ID: 559292)
Method Details:
Experimental Method:
Resolution:
2.80 Å
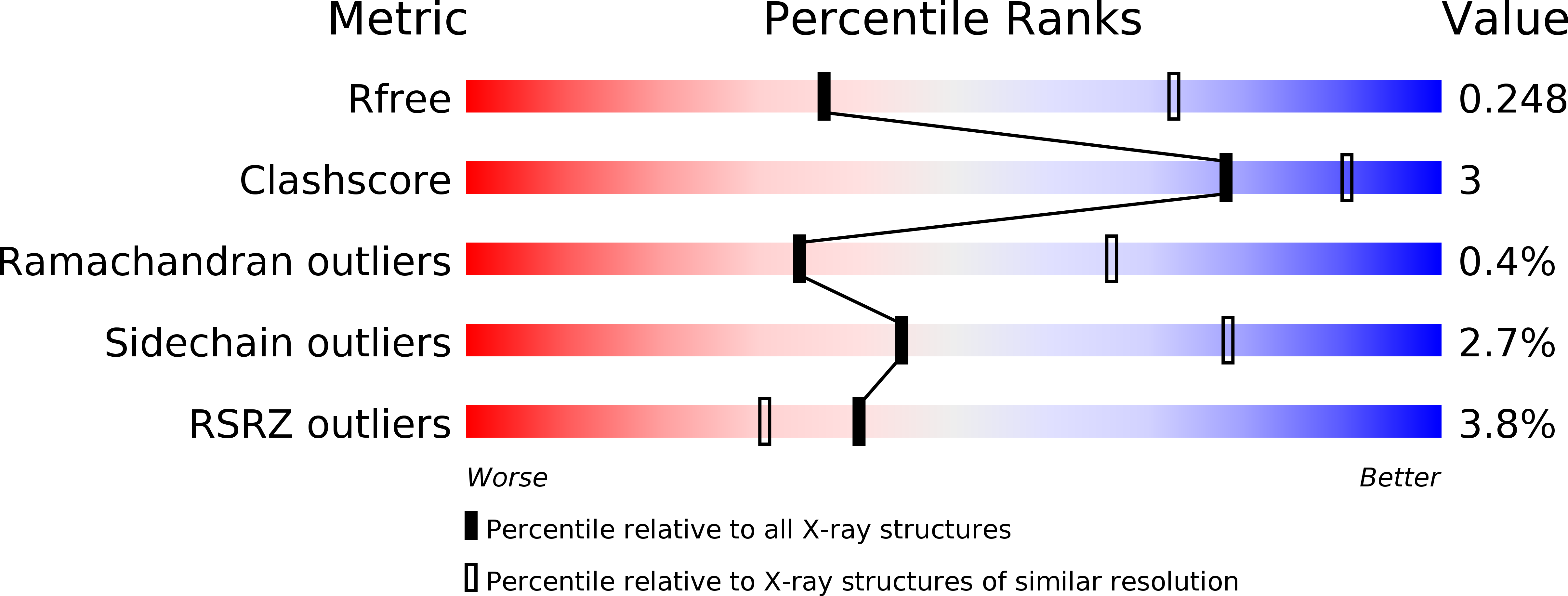
R-Value Free:
0.24
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
P 1 21 1