Deposition Date
2013-03-07
Release Date
2013-08-14
Last Version Date
2025-07-23
Entry Detail
PDB ID:
4JIV
Keywords:
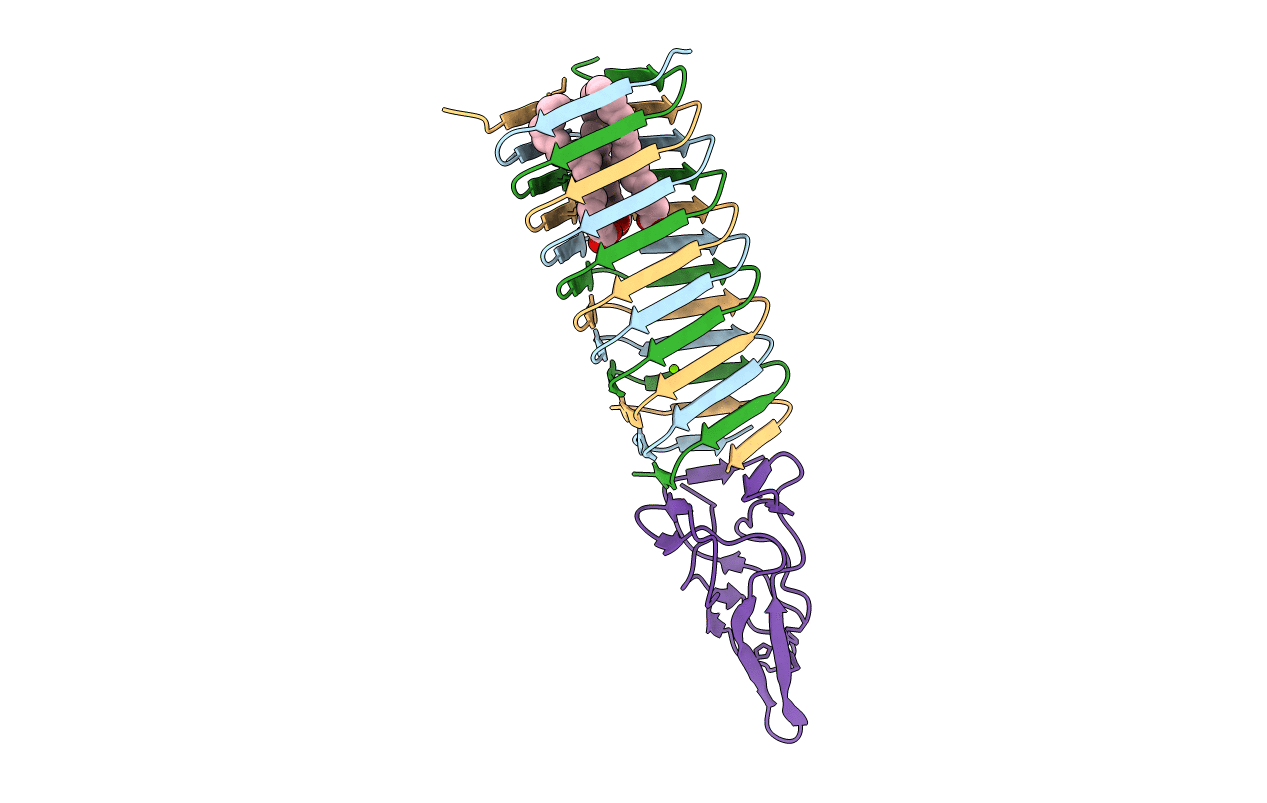
Title:
VCA0105 PAAR-repeat protein from Vibrio cholerae in complex with a VgrG-like beta-helix that is based on a fragment of T4 gp5
Biological Source:
Source Organism(s):
Enterobacteria phage T4 (Taxon ID: 10665)
Vibrio cholerae O1 biovar eltor (Taxon ID: 243277)
Vibrio cholerae O1 biovar eltor (Taxon ID: 243277)
Expression System(s):
Method Details:
Experimental Method:
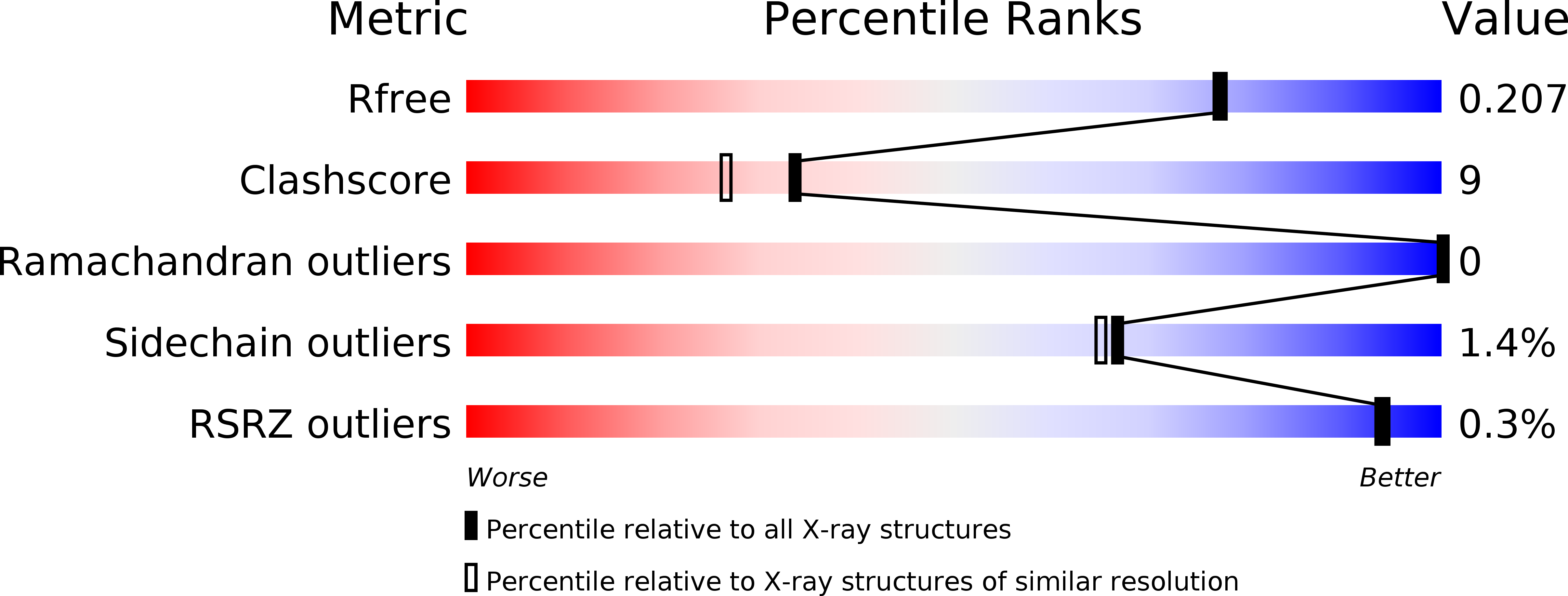
Resolution:
1.90 Å
R-Value Free:
0.20
R-Value Work:
0.16
R-Value Observed:
0.16
Space Group:
P 42 2 2