Deposition Date
2012-12-22
Release Date
2013-03-27
Last Version Date
2024-03-20
Entry Detail
PDB ID:
4IJQ
Keywords:
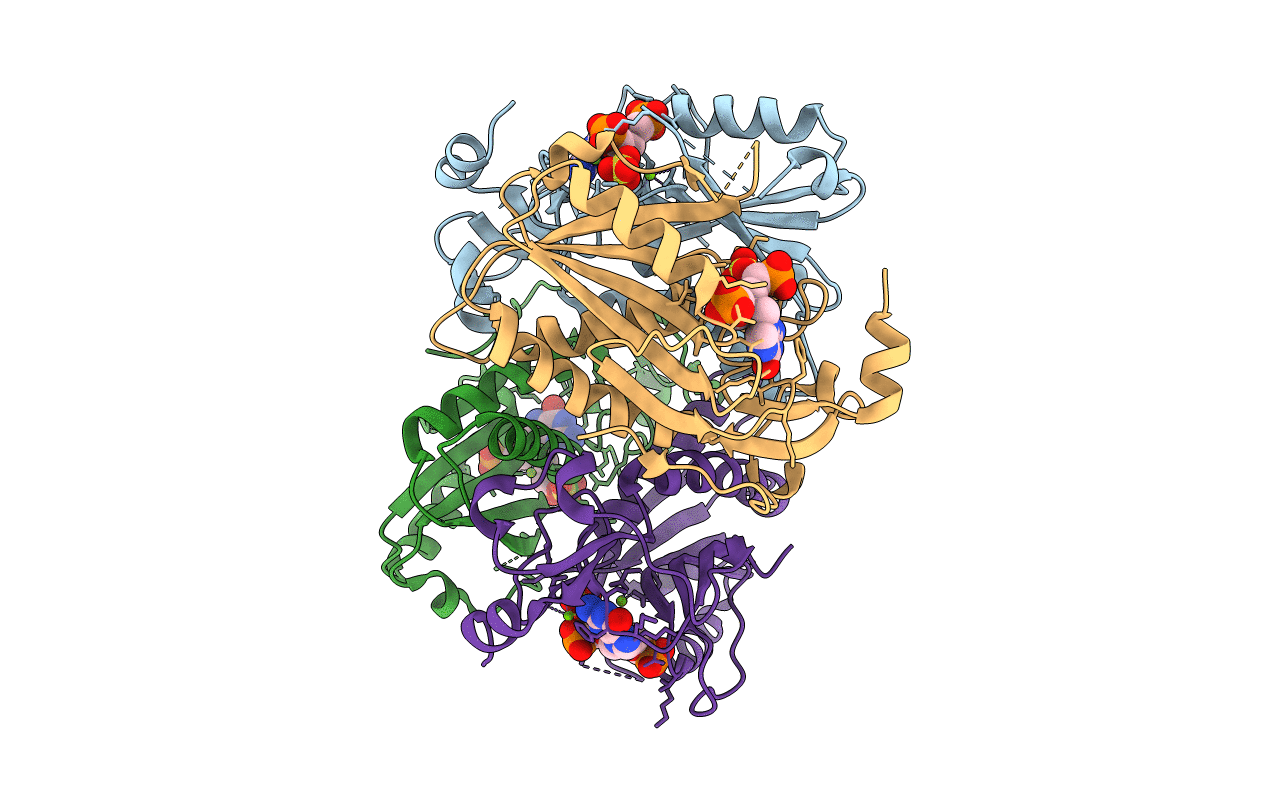
Title:
Human hypoxanthine-guanine phosphoribosyltransferase in complex with [(2-((Guanine-9H-yl)methyl)propane-1,3-diyl)bis(oxy)]bis(methylene))diphosphonic acid
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.00 Å
R-Value Free:
0.22
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 1 21 1


