Deposition Date
2012-11-28
Release Date
2013-05-08
Last Version Date
2023-11-08
Entry Detail
PDB ID:
4I4Z
Keywords:
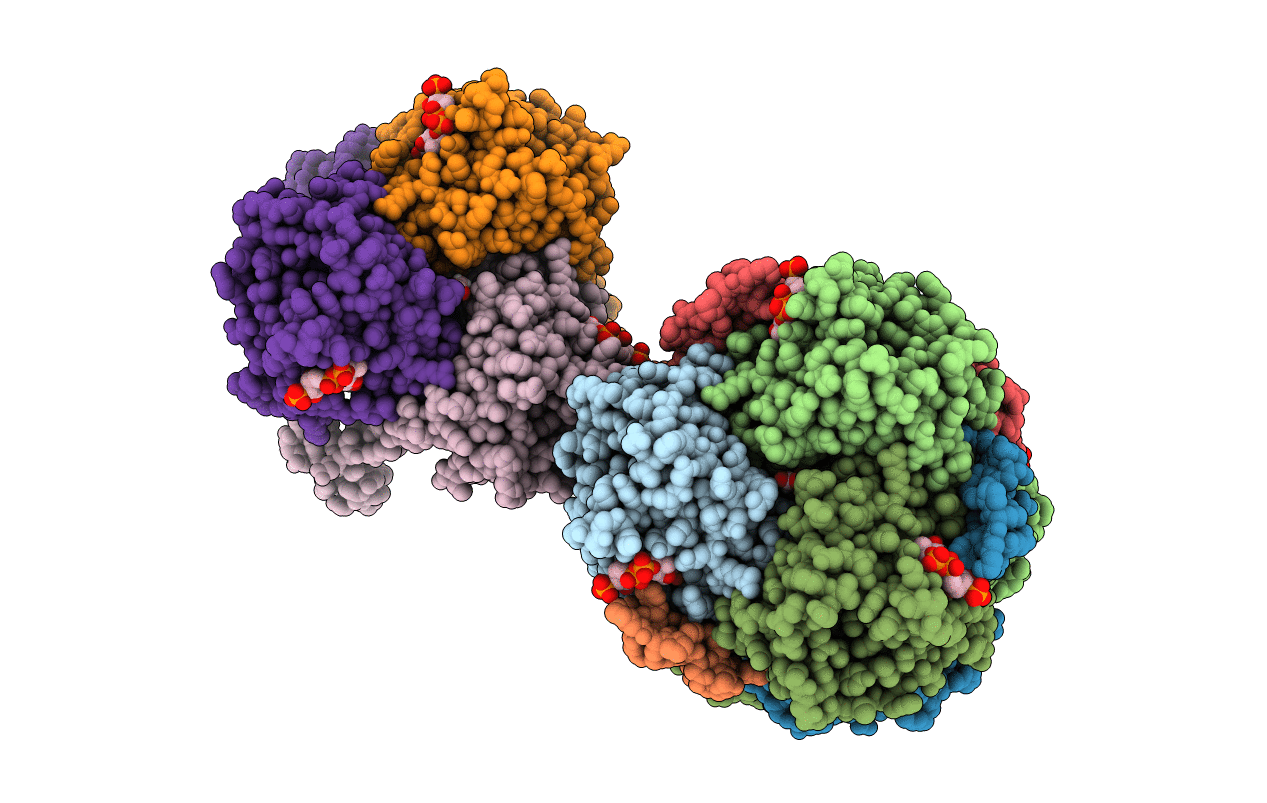
Title:
Synechocystis sp. PCC 6803 1,4-dihydroxy-2-naphthoyl-coenzyme A synthase (MenB) in complex with salicylyl-CoA
Biological Source:
Source Organism(s):
Synechocystis sp. (Taxon ID: 1111708)
Expression System(s):
Method Details:
Experimental Method:
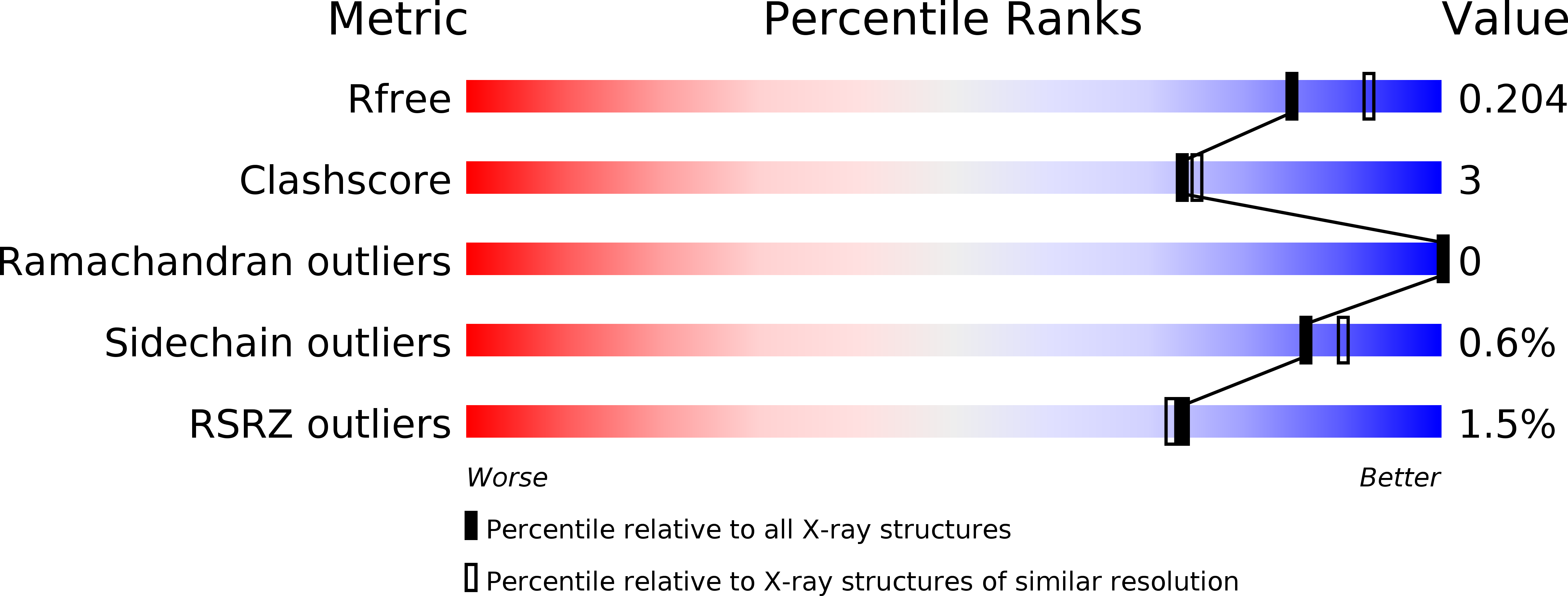
Resolution:
2.00 Å
R-Value Free:
0.22
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 32 2 1