Deposition Date
2012-11-15
Release Date
2013-05-01
Last Version Date
2024-11-20
Entry Detail
PDB ID:
4HZL
Keywords:
Title:
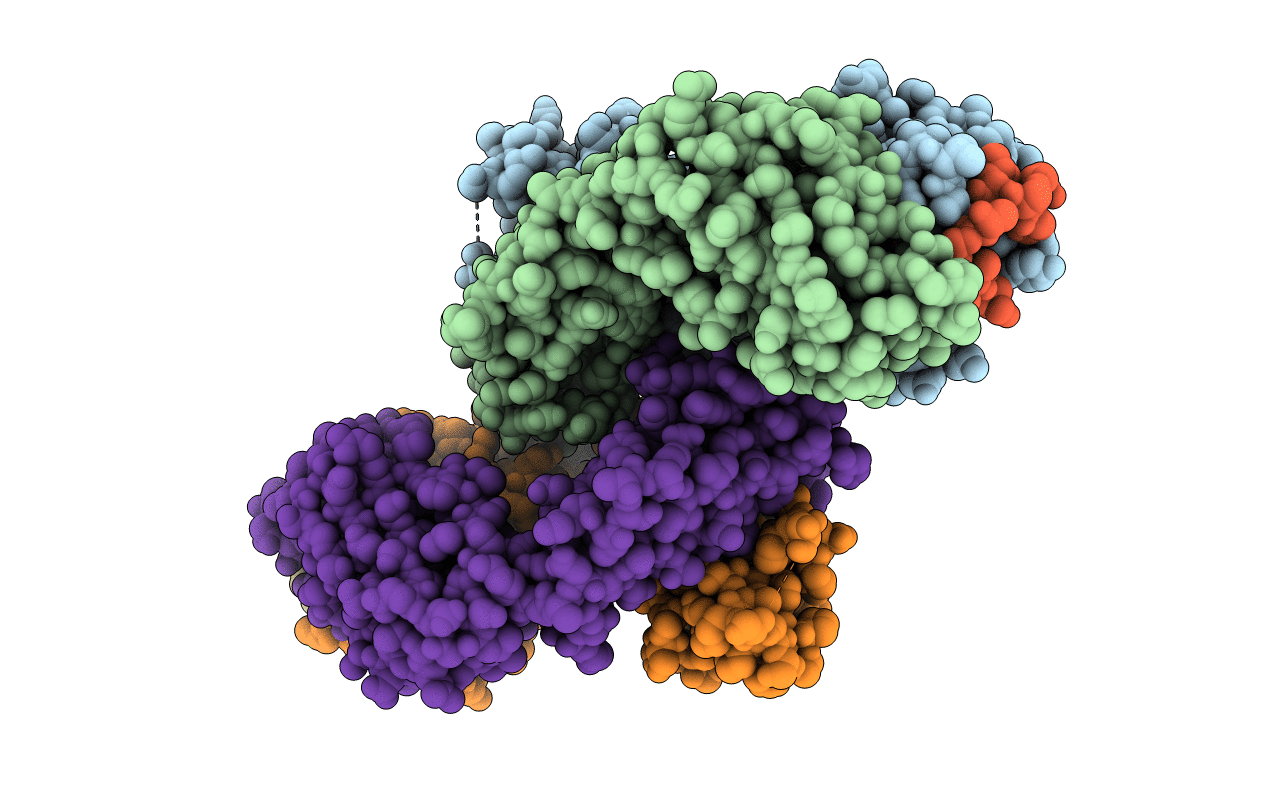
Neutralizing antibody mAb#8 in complex with the Epitope II of HCV E2 envelope protein
Biological Source:
Source Organism(s):
Mus musculus (Taxon ID: 10090)
Recombinant Hepatitis C virus H77(5'UTR-NS2)/JFH1_V787A,Q1247L (Taxon ID: 1006431)
Recombinant Hepatitis C virus H77(5'UTR-NS2)/JFH1_V787A,Q1247L (Taxon ID: 1006431)
Method Details:
Experimental Method:
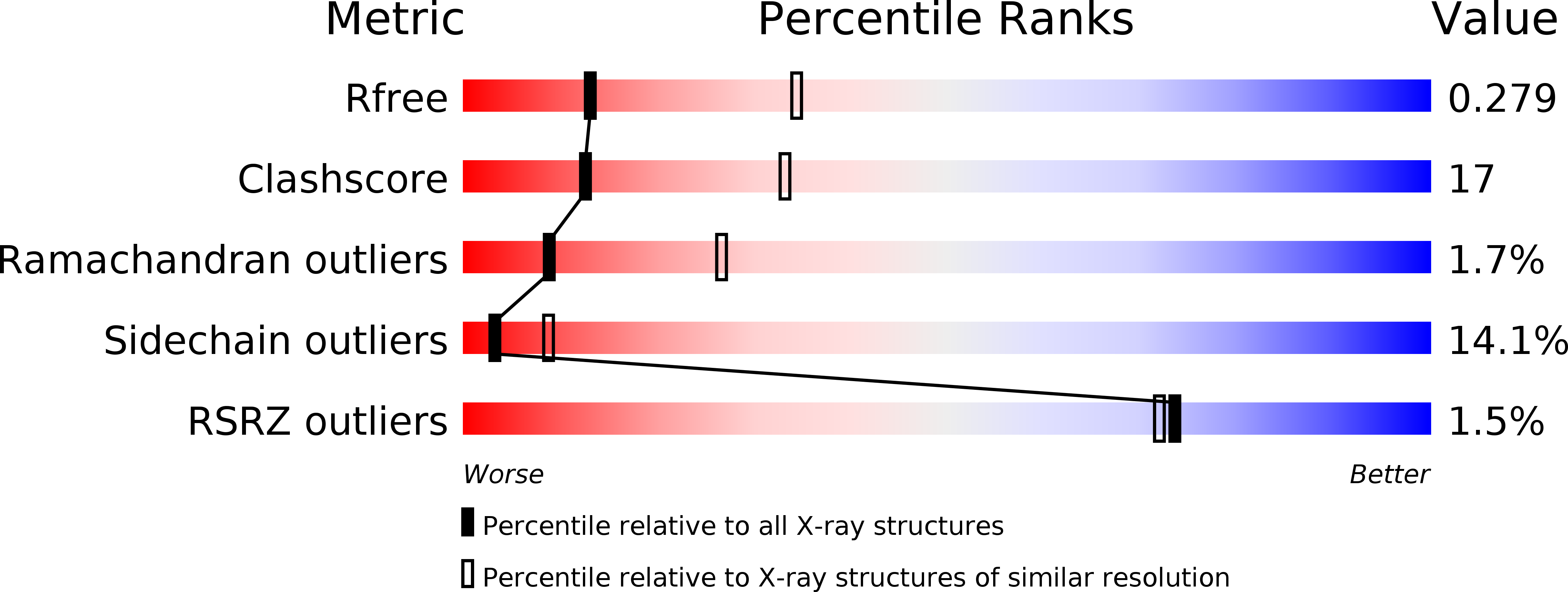
Resolution:
2.85 Å
R-Value Free:
0.27
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
P 43 21 2