Deposition Date
2012-10-15
Release Date
2014-01-08
Last Version Date
2024-02-28
Entry Detail
PDB ID:
4HK9
Keywords:
Title:
Crystal Structures of Mutant Endo-beta-1,4-xylanase II Complexed with substrate (1.15 A) and Products (1.6 A)
Biological Source:
Source Organism:
Trichoderma reesei (Taxon ID: 51453)
Host Organism:
Method Details:
Experimental Method:
Resolution:
1.55 Å
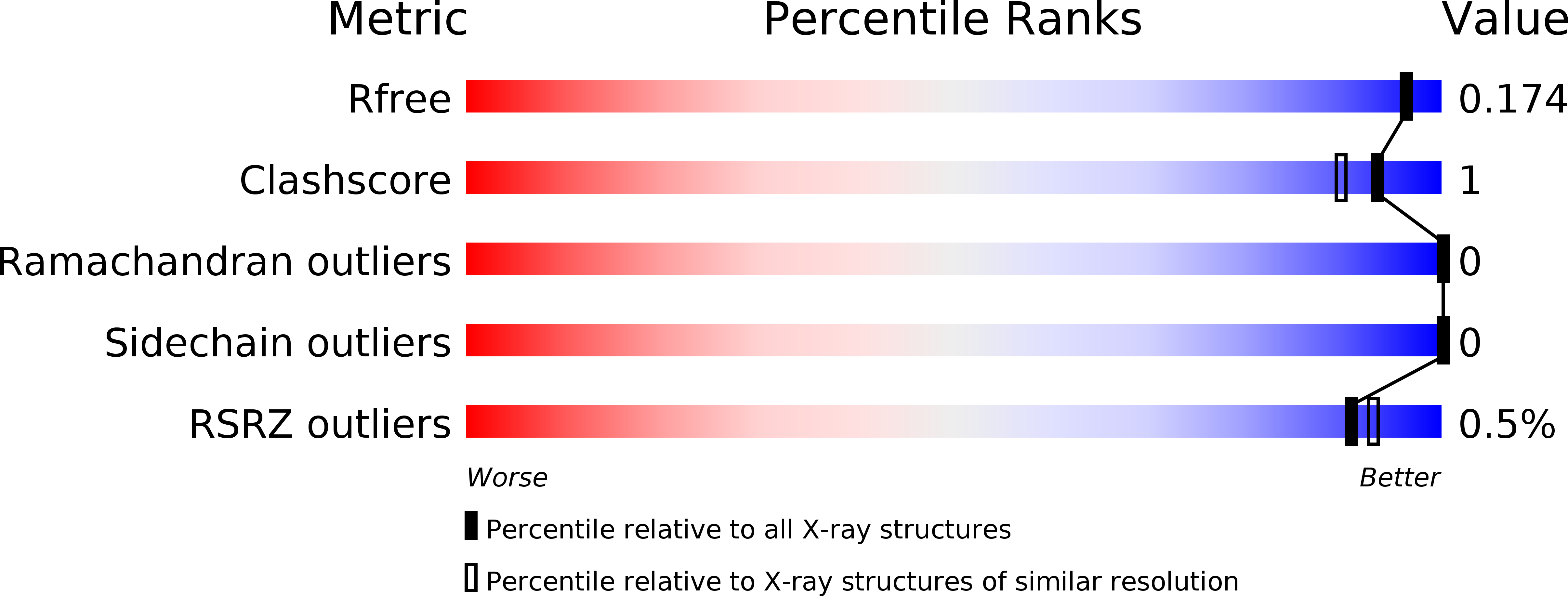
R-Value Free:
0.17
R-Value Work:
0.14
R-Value Observed:
0.15
Space Group:
P 21 21 21