Deposition Date
2012-09-13
Release Date
2013-03-06
Last Version Date
2025-03-26
Entry Detail
PDB ID:
4H2X
Keywords:
Title:
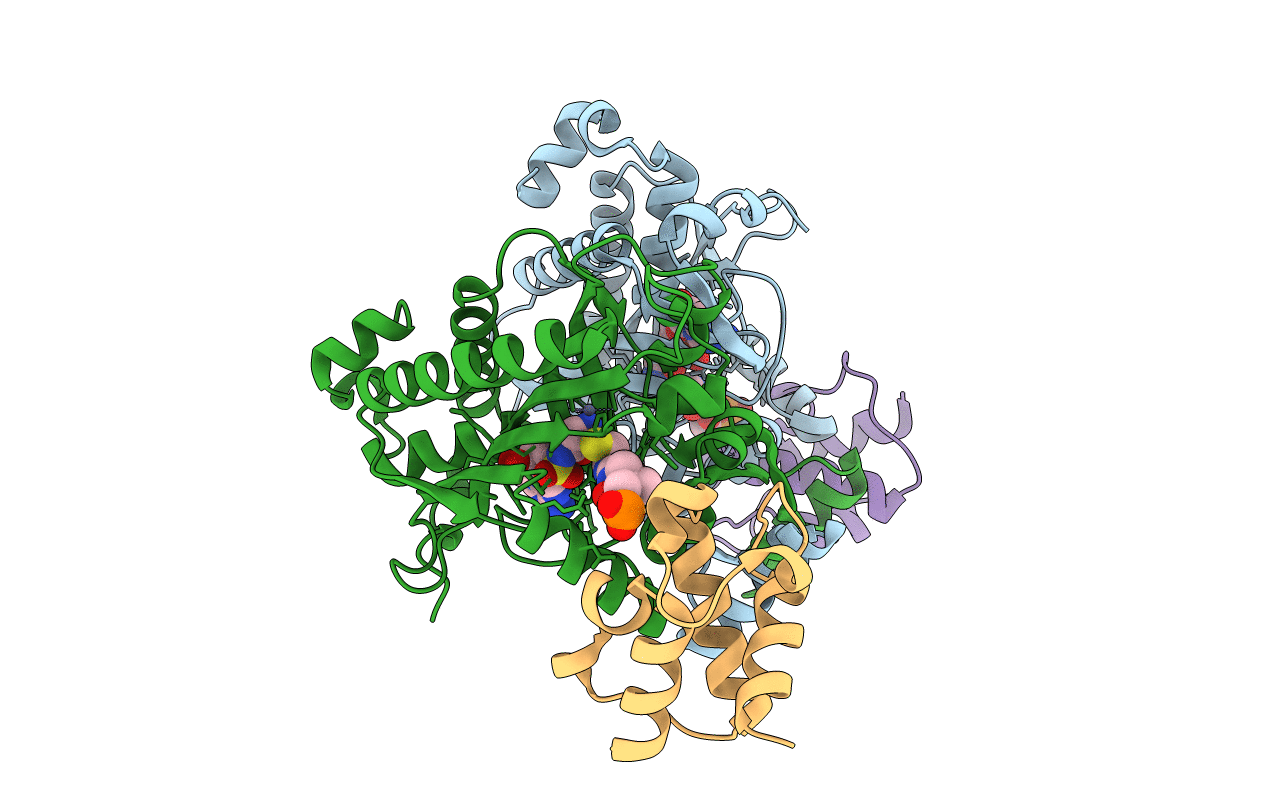
Crystal structure of engineered Bradyrhizobium japonicum glycine:[carrier protein] ligase complexed with carrier protein from Agrobacterium tumefaciens and an analogue of glycyl adenylate
Biological Source:
Source Organism(s):
Bradyrhizobium japonicum (Taxon ID: 224911)
Agrobacterium fabrum (strain C58 / ATCC 33970) (Taxon ID: 176299)
Agrobacterium fabrum (strain C58 / ATCC 33970) (Taxon ID: 176299)
Expression System(s):
Method Details:
Experimental Method:
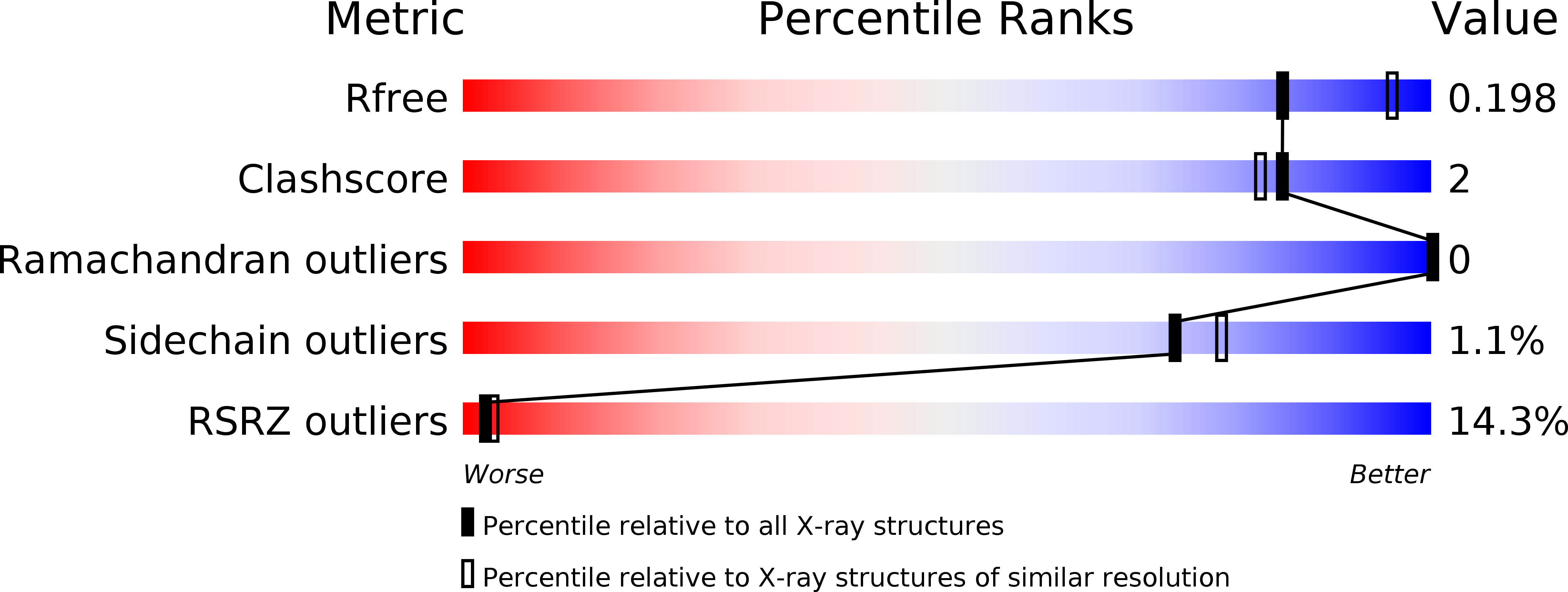
Resolution:
2.15 Å
R-Value Free:
0.19
R-Value Work:
0.16
R-Value Observed:
0.16
Space Group:
P 21 21 21