Deposition Date
2012-08-28
Release Date
2013-02-27
Last Version Date
2024-03-20
Entry Detail
PDB ID:
4GSR
Keywords:
Title:
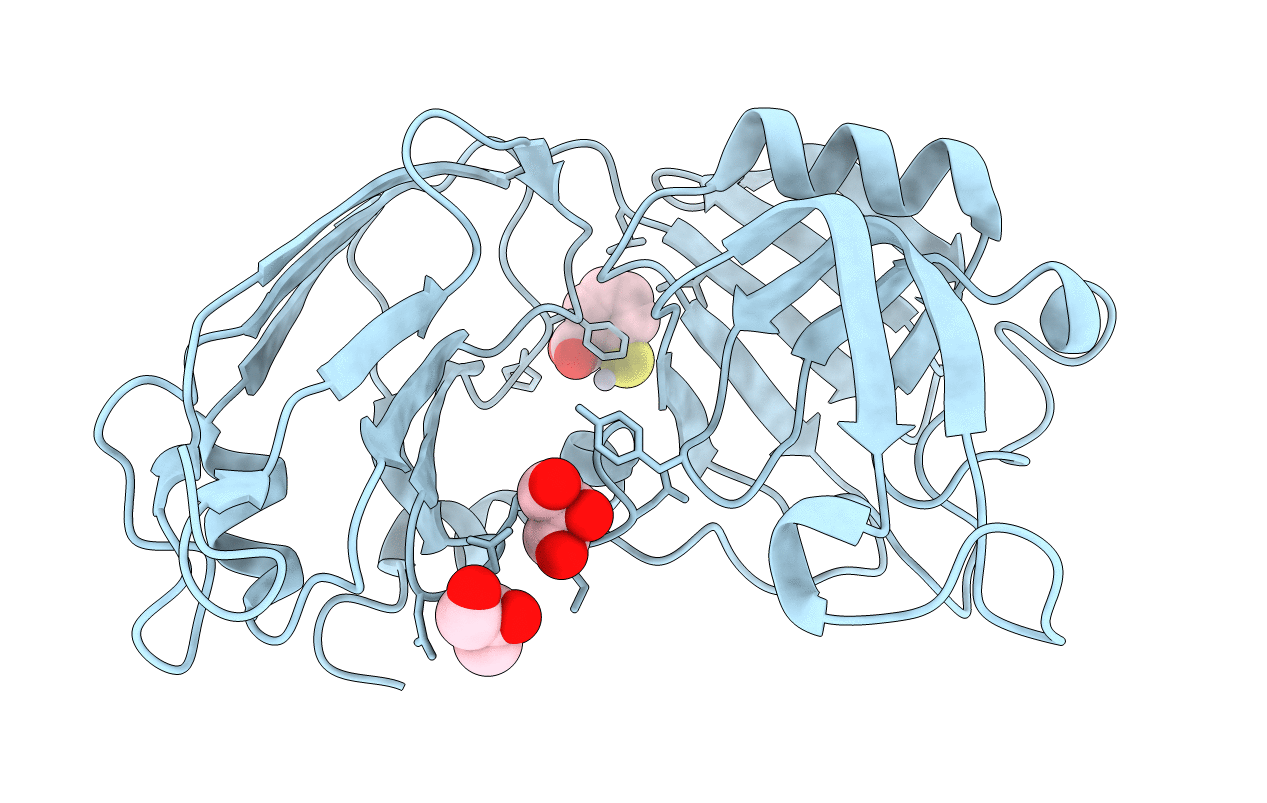
Structural basis for the inhibition of Mycobacterium tuberculosis L,D-transpeptidase by meropenem, a drug effective against extensively drug-resistant strains
Biological Source:
Source Organism(s):
Mycobacterium tuberculosis (Taxon ID: 1773)
Expression System(s):
Method Details:
Experimental Method:
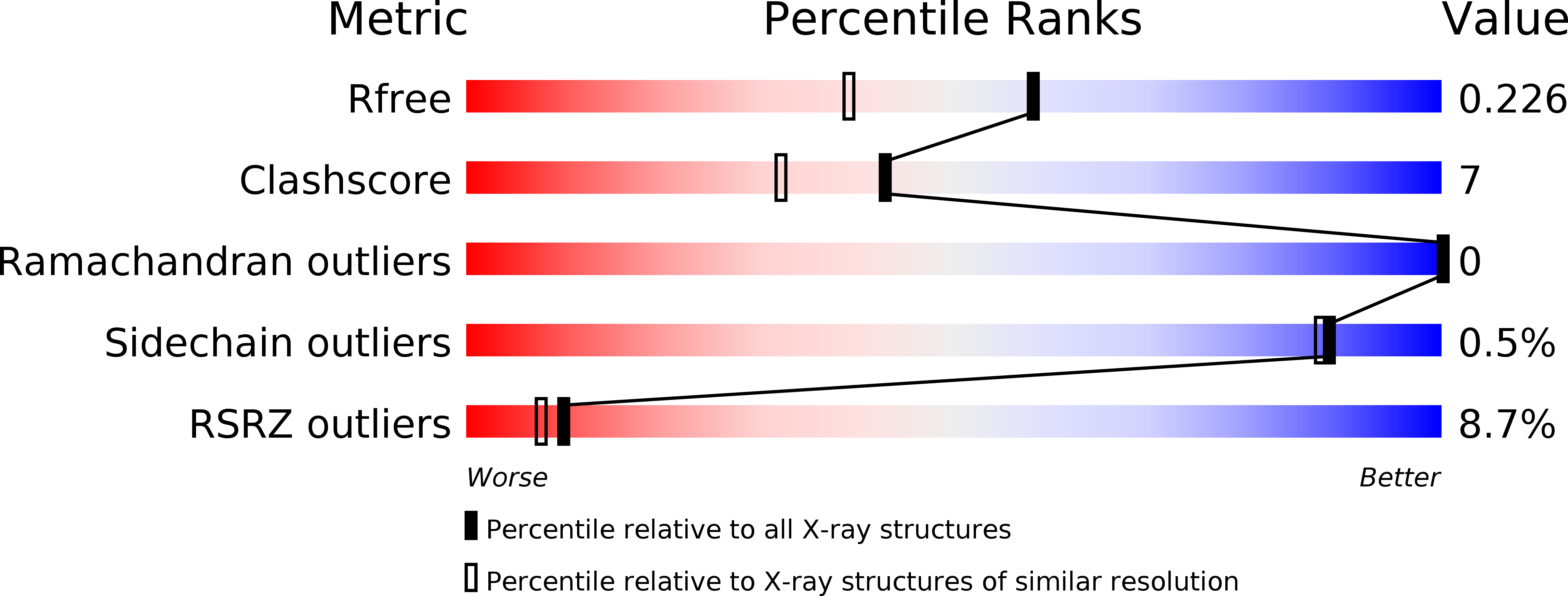
Resolution:
1.79 Å
R-Value Free:
0.22
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
C 1 2 1