Deposition Date
2012-08-27
Release Date
2013-08-28
Last Version Date
2023-09-13
Entry Detail
PDB ID:
4GS8
Keywords:
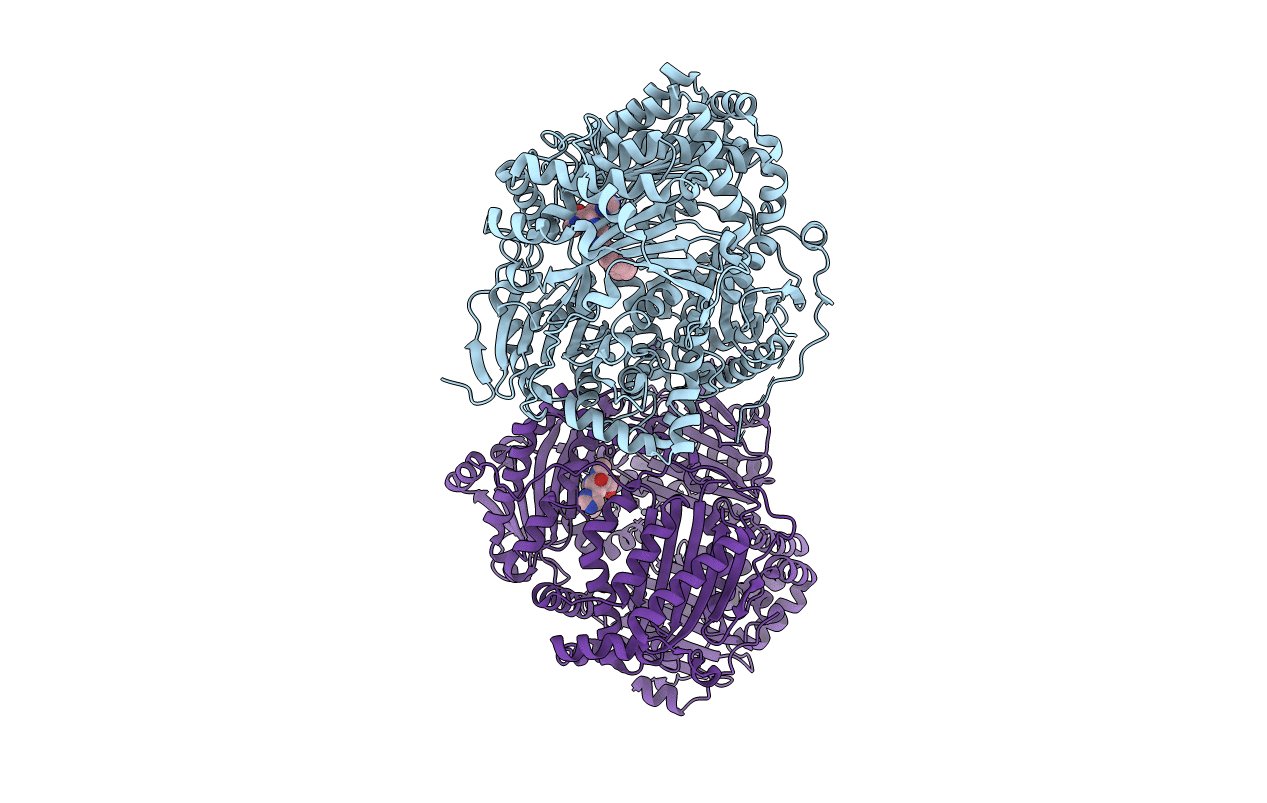
Title:
Structure analysis of cysteine free insulin degrading enzyme (ide) with compound bdm43079 [{[(s)-2-(1h-imidazol-4-yl)-1-methylcarbamoyl-ethylcarbamoyl]-methyl}-(3-phenyl-propyl)-amino]-acetic acid
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
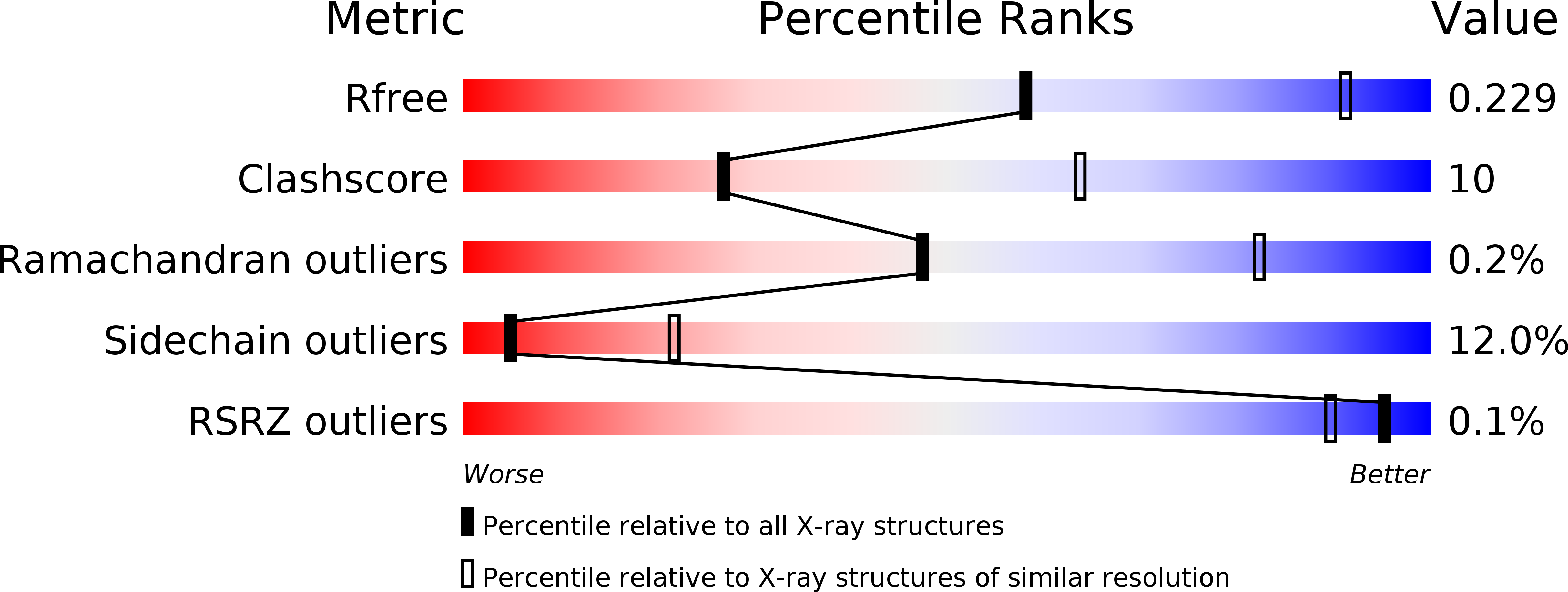
Resolution:
2.99 Å
R-Value Free:
0.23
R-Value Work:
0.17
R-Value Observed:
0.18
Space Group:
P 65