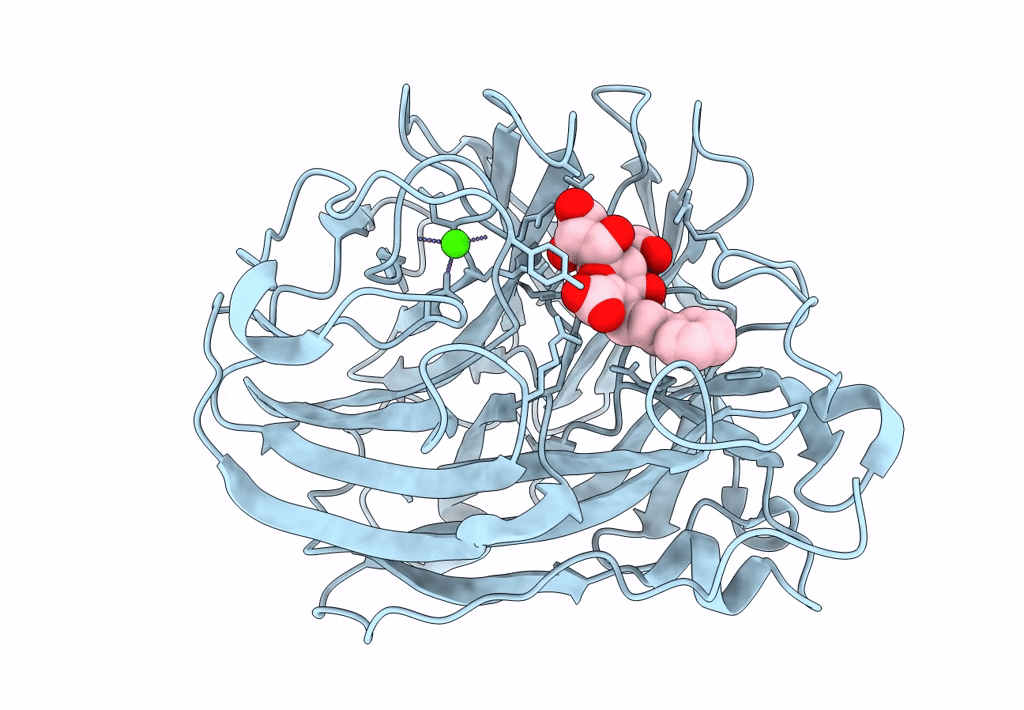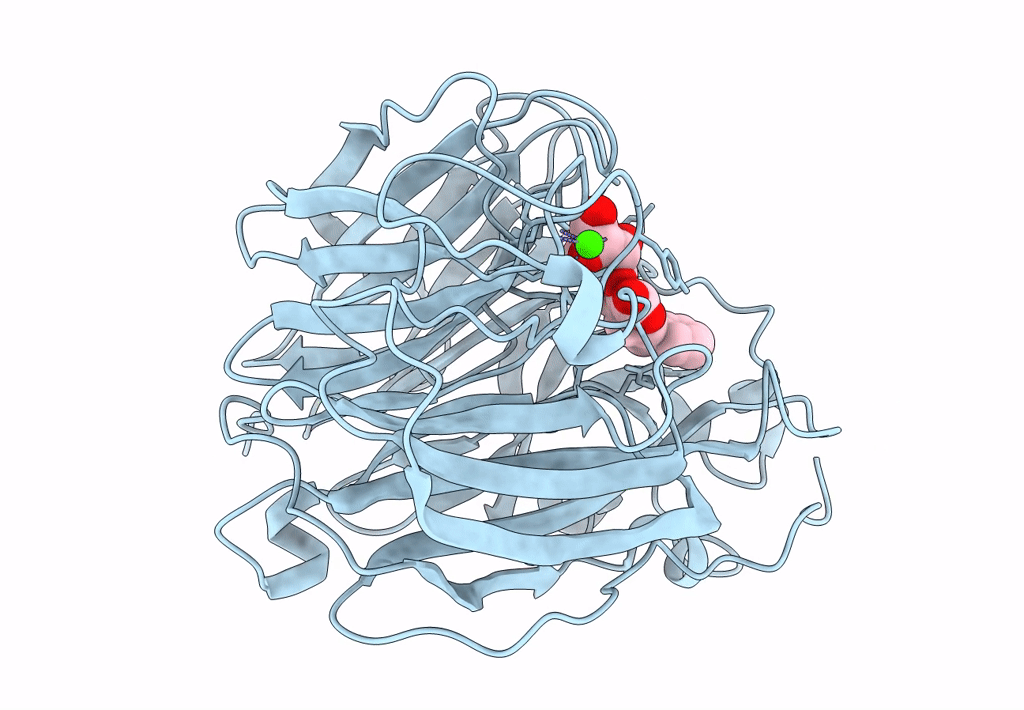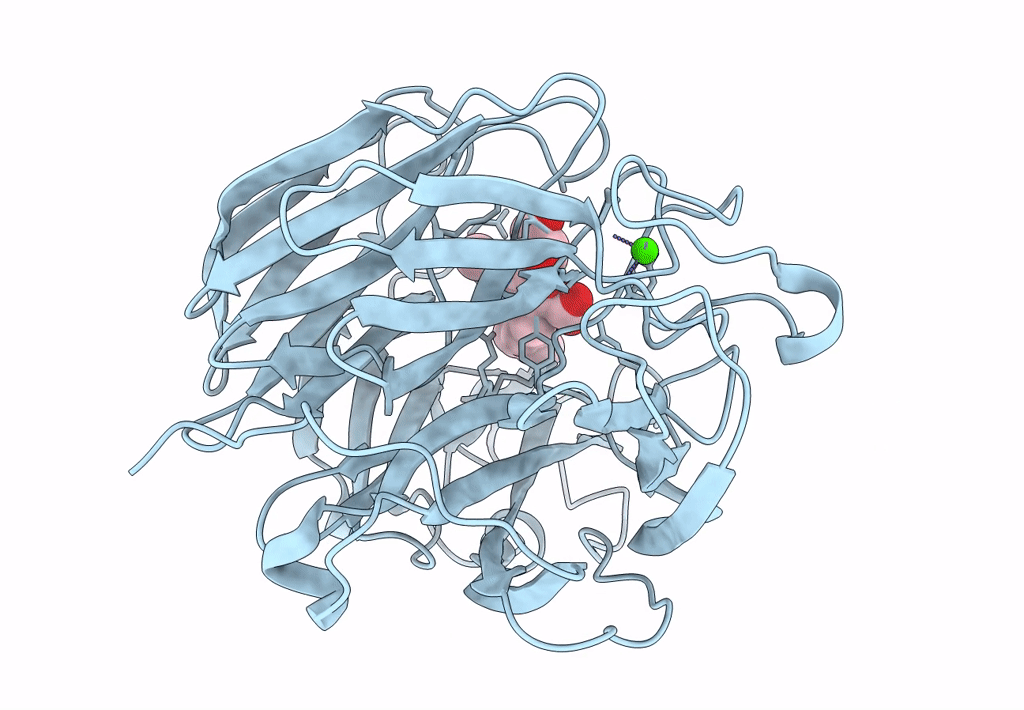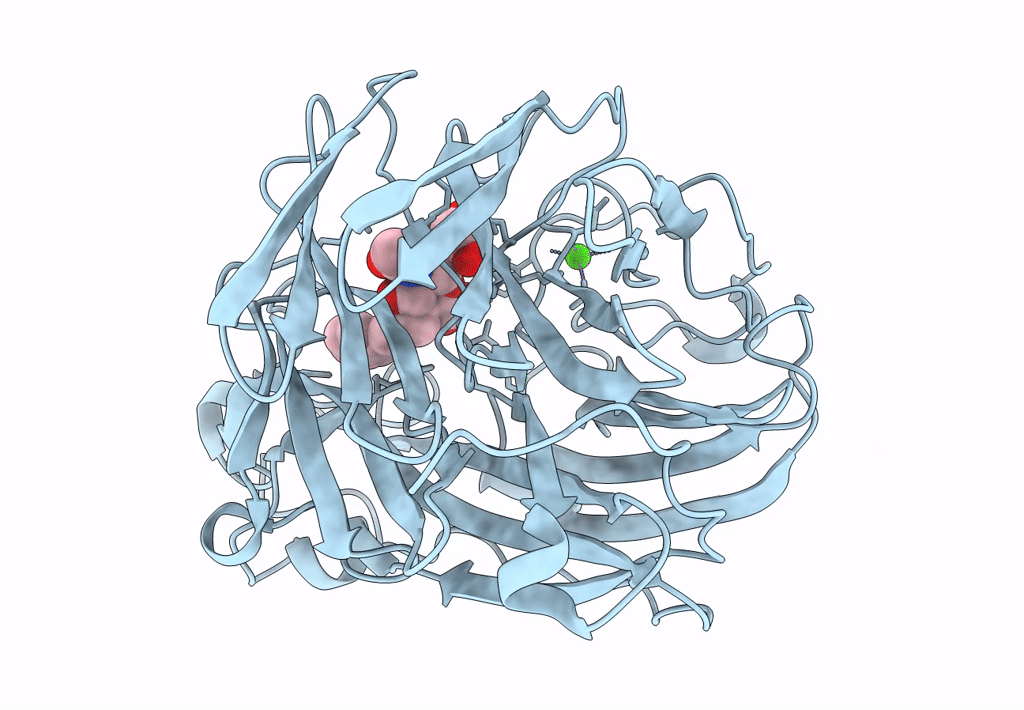
Deposition Date
2012-07-26
Release Date
2012-09-26
Last Version Date
2024-10-30
Entry Detail
PDB ID:
4GB1
Keywords:
Title:
Synthesis and Evaluation of Novel 3-C-alkylated-Neu5Ac2en Derivatives as Probes of Influenza Virus Sialidase 150-loop flexibility
Biological Source:
Source Organism(s):
Influenza A virus (Taxon ID: 385580)
Method Details:
Experimental Method:
Resolution:
2.62 Å
R-Value Free:
0.27
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
I 4


