Deposition Date
2012-07-18
Release Date
2012-10-10
Last Version Date
2023-09-13
Entry Detail
PDB ID:
4G63
Keywords:
Title:
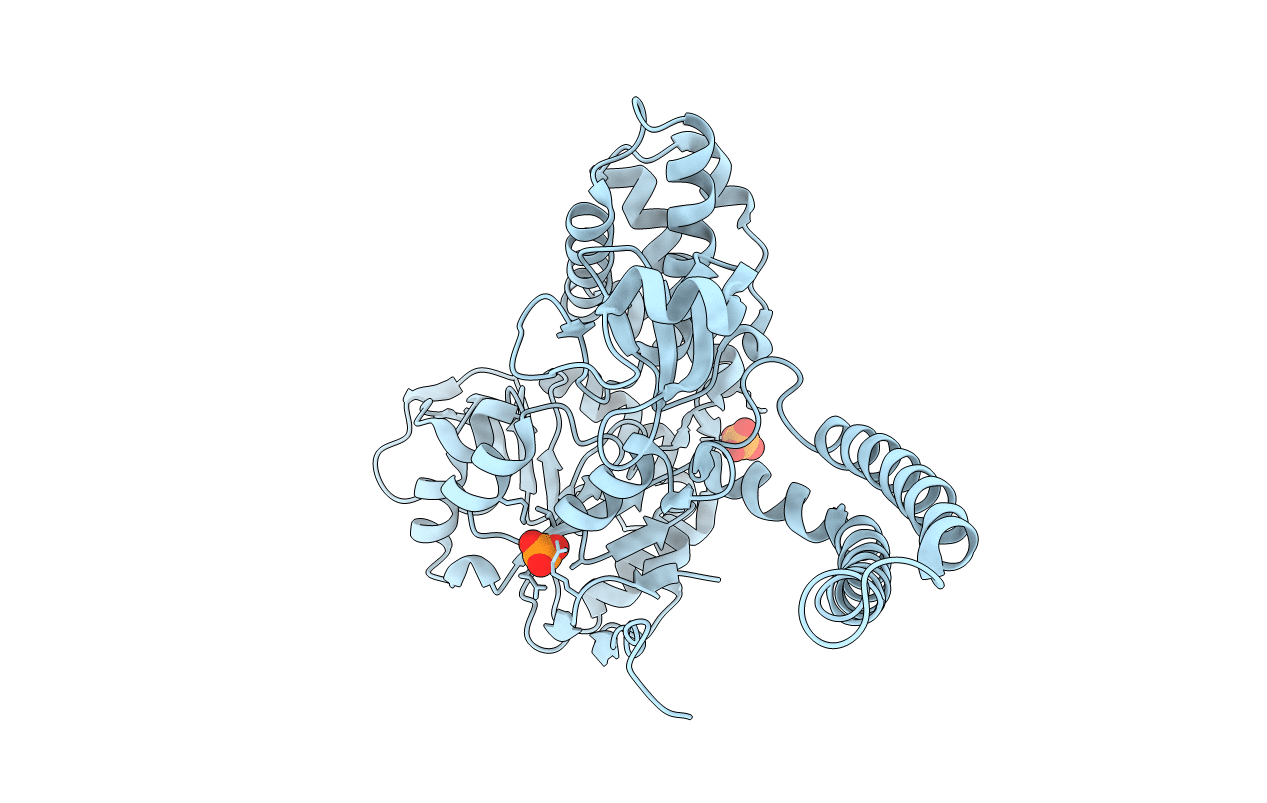
Crystal structure of cytosolic IMP-GMP specific 5'-nucleotidase (lpg0095) in complex with phosphate ions from Legionella pneumophila, Northeast Structural Genomics Consortium Target LgR1
Biological Source:
Source Organism(s):
Legionella pneumophila subsp. pneumophila (Taxon ID: 272624)
Expression System(s):
Method Details:
Experimental Method:
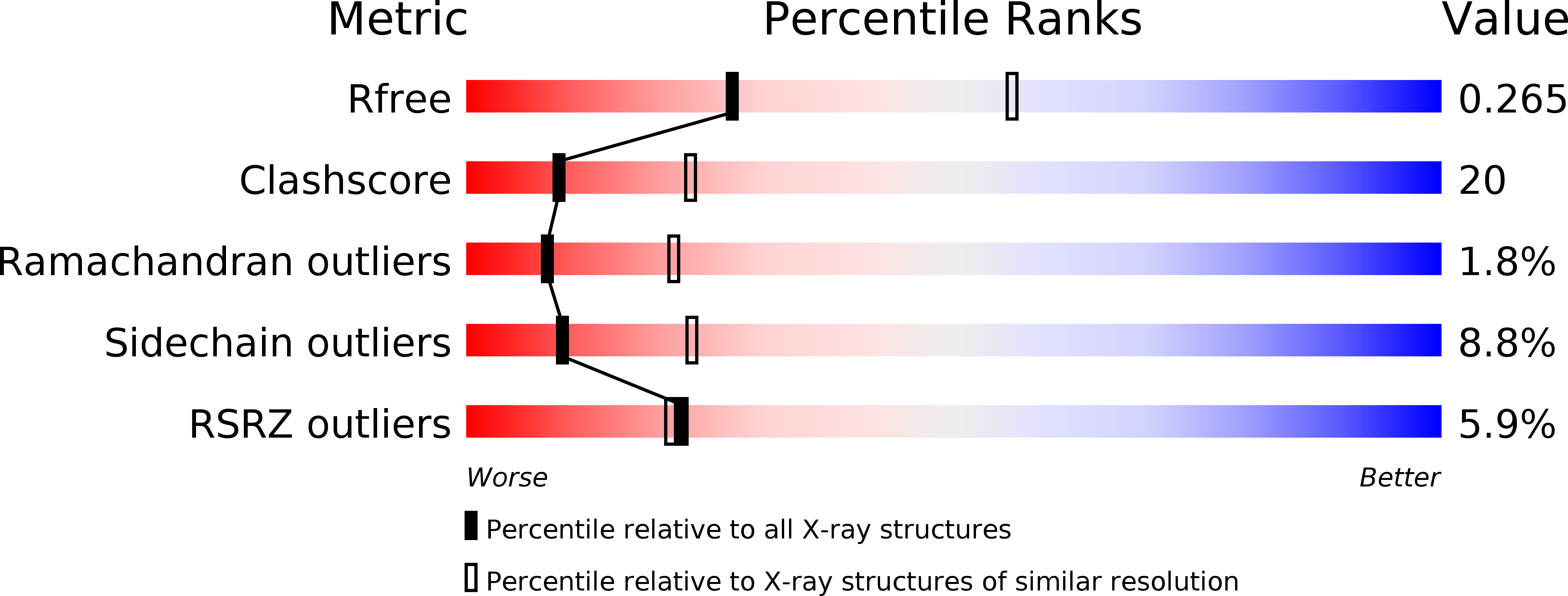
Resolution:
2.70 Å
R-Value Free:
0.25
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
I 41 2 2