Deposition Date
2012-07-14
Release Date
2013-07-17
Last Version Date
2023-09-13
Entry Detail
PDB ID:
4G3J
Keywords:
Title:
Sterol 14-alpha demethylase (CYP51) from Trypanosoma brucei in complex with the VNI derivative (R)-N-(1-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-yl)ethyl)-4-(5-phenyl-1,3,4-oxadiazol-2-yl)benzamide [R-VNI-triazole (VNT)]
Biological Source:
Source Organism(s):
Trypanosoma brucei (Taxon ID: 5691)
Expression System(s):
Method Details:
Experimental Method:
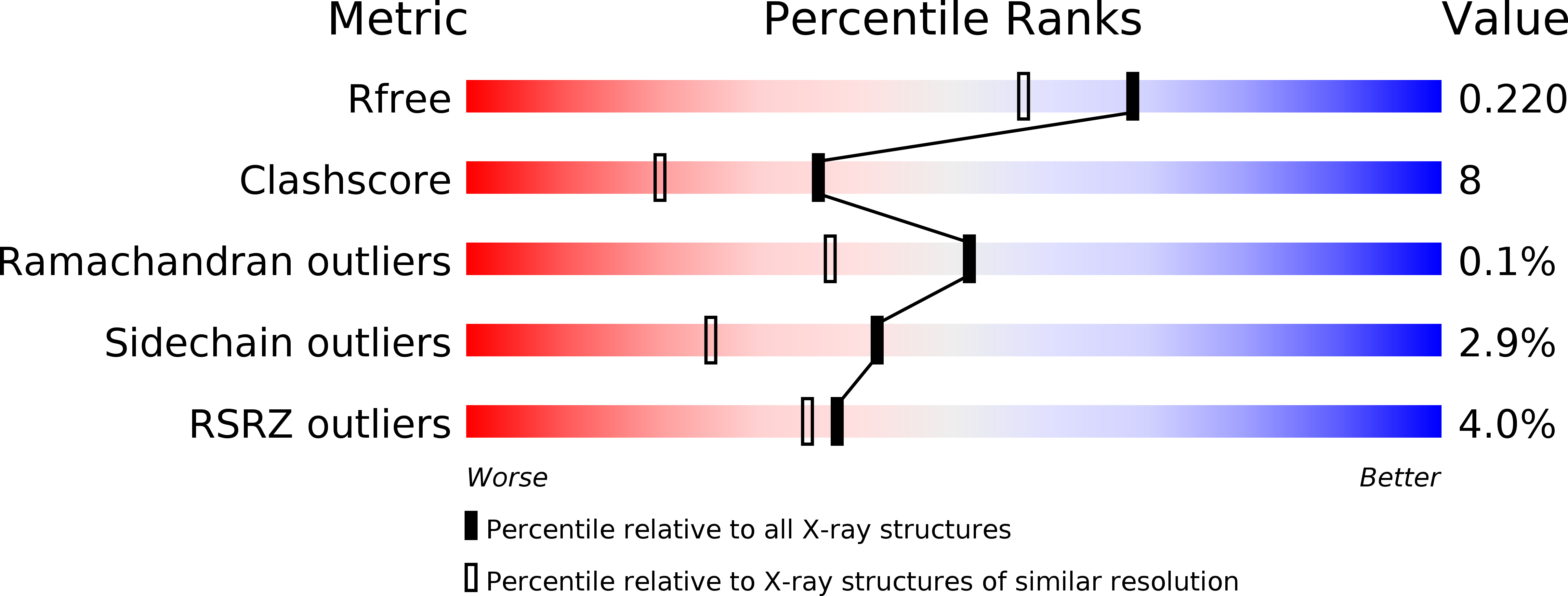
Resolution:
1.83 Å
R-Value Free:
0.22
R-Value Work:
0.16
R-Value Observed:
0.17
Space Group:
P 1