Deposition Date
2012-06-27
Release Date
2012-07-11
Last Version Date
2024-02-28
Entry Detail
PDB ID:
4FS8
Keywords:
Title:
The structure of an As(III) S-adenosylmethionine methyltransferase: insights into the mechanism of arsenic biotransformation
Biological Source:
Source Organism(s):
Cyanidioschyzon sp. 5508 (Taxon ID: 610260)
Expression System(s):
Method Details:
Experimental Method:
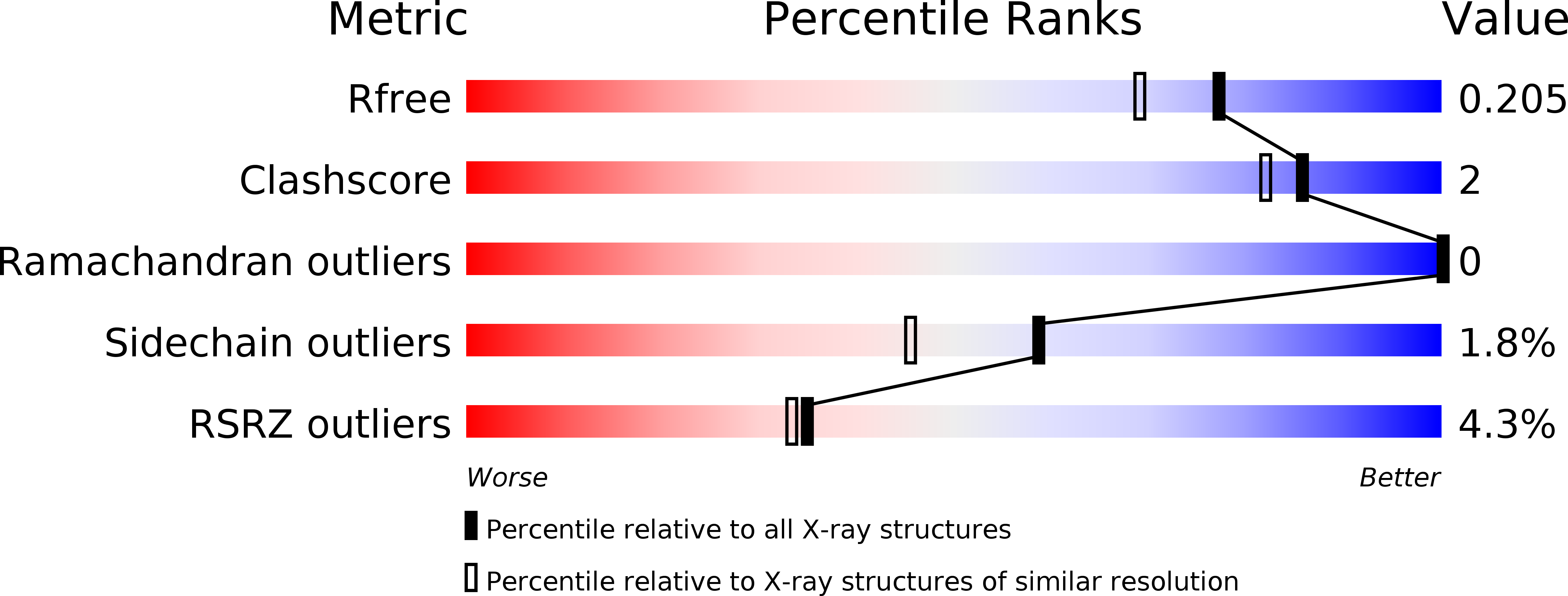
Resolution:
1.78 Å
R-Value Free:
0.20
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
C 1 2 1