Deposition Date
2012-05-30
Release Date
2013-02-27
Last Version Date
2024-02-28
Entry Detail
PDB ID:
4FEO
Keywords:
Title:
Crystal structure of the AU25A/A46G/C74U mutant xpt-pbuX guanine riboswitch aptamer domain in complex with 2,6-diaminopurine
Method Details:
Experimental Method:
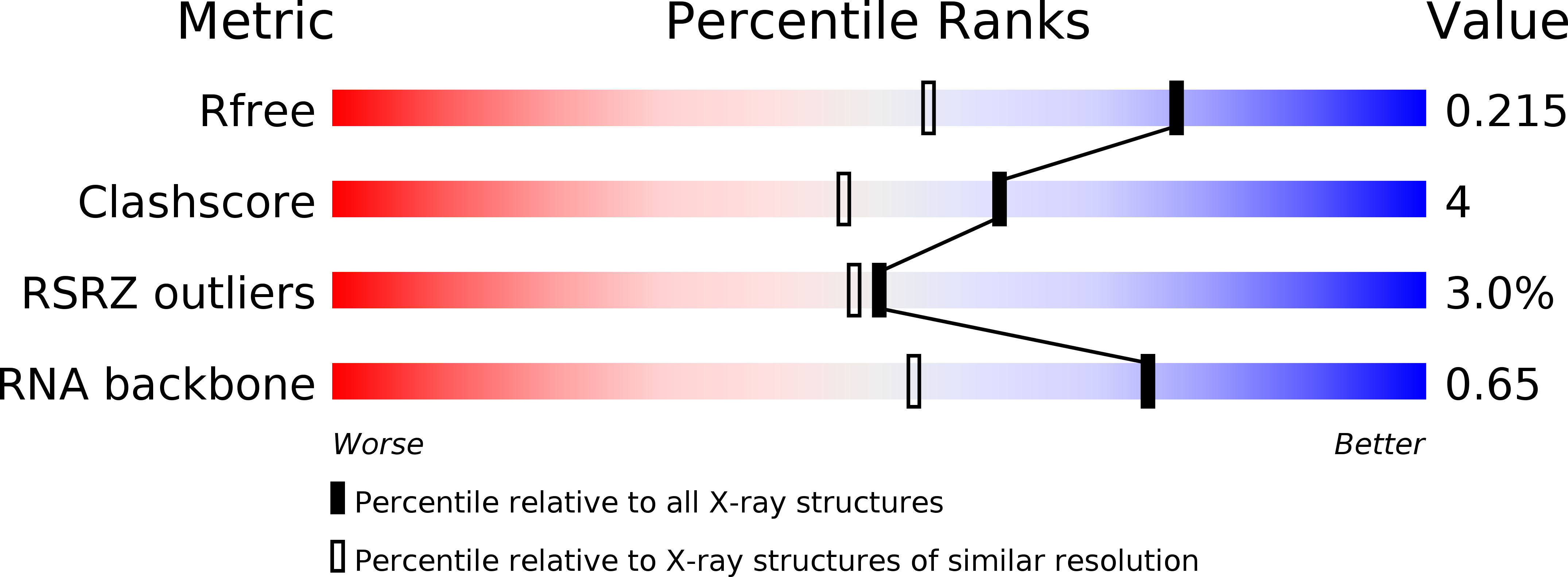
Resolution:
1.60 Å
R-Value Free:
0.22
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
C 1 2 1