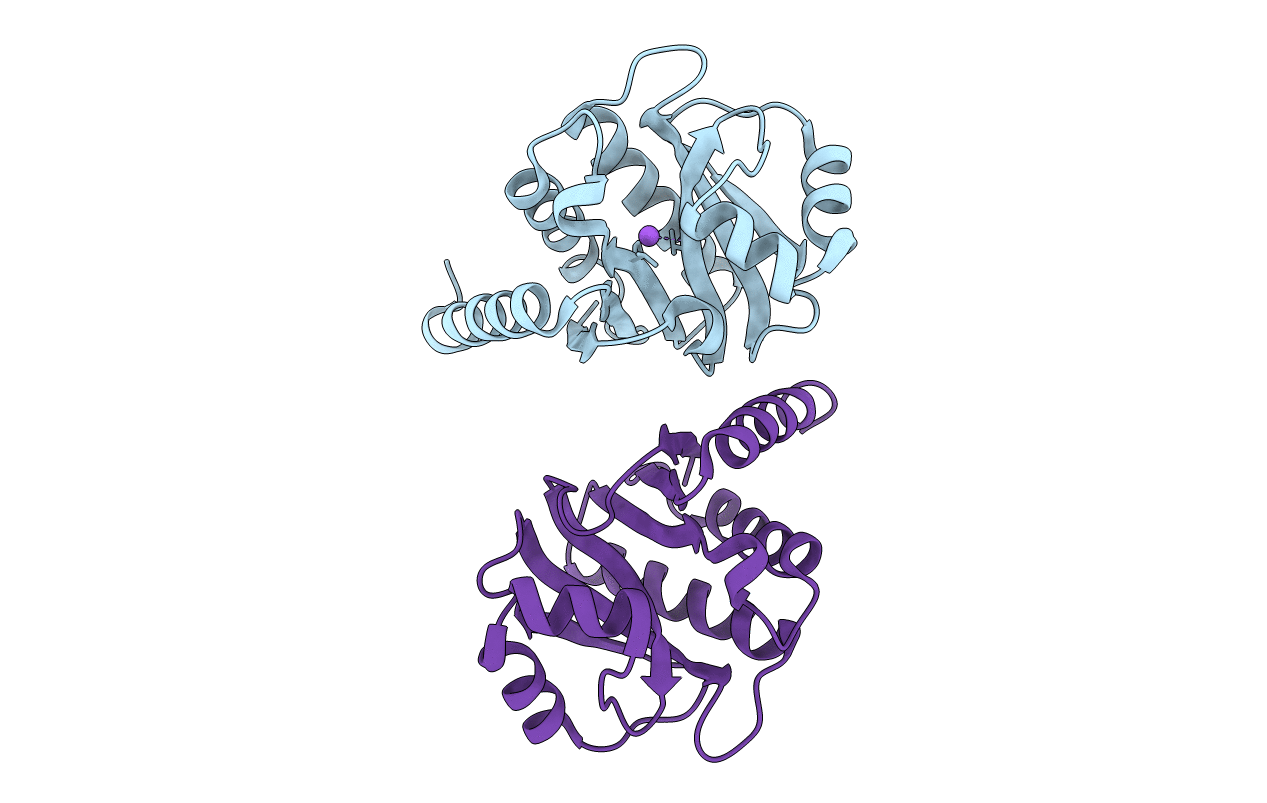
Deposition Date
2012-04-24
Release Date
2012-06-13
Last Version Date
2024-11-06
Entry Detail
PDB ID:
4ETK
Keywords:
Title:
Crystal Structure of E6A/L130D/A155H variant of de novo designed serine hydrolase, Northeast Structural Genomics Consortium (NESG) Target OR186
Biological Source:
Source Organism(s):
artificial gene (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
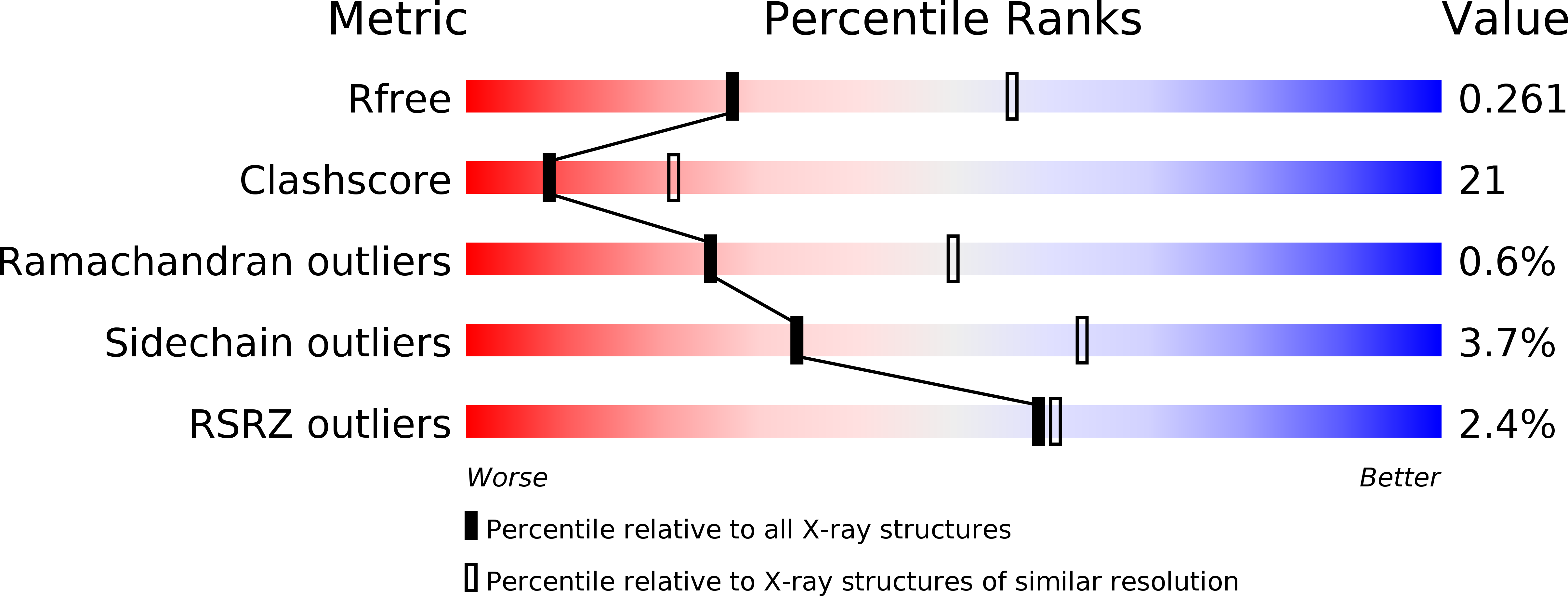
Resolution:
2.70 Å
R-Value Free:
0.25
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
P 21 21 2