Deposition Date
2012-03-20
Release Date
2012-07-04
Last Version Date
2024-02-28
Entry Detail
PDB ID:
4E8F
Keywords:
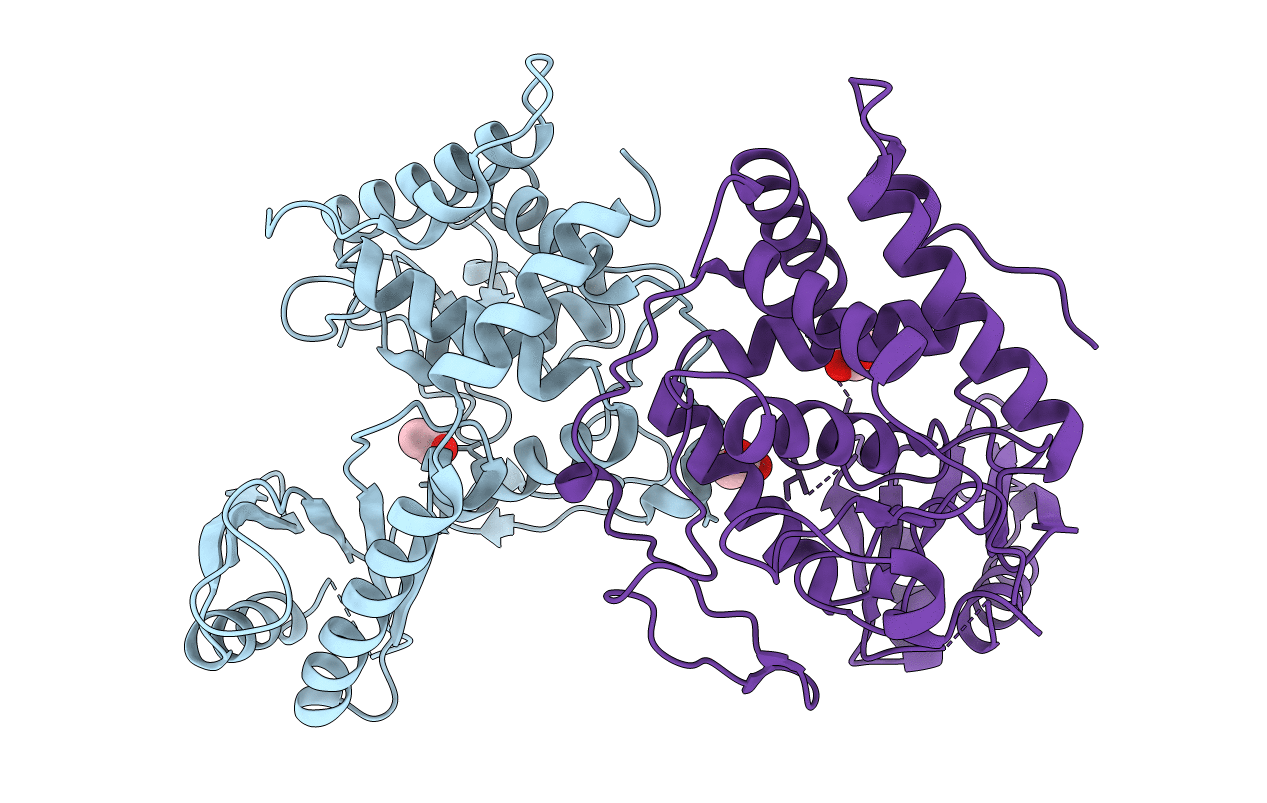
Title:
Structural Basis for the Activity of a Cytoplasmic RNA Terminal U-transferase
Biological Source:
Source Organism(s):
Schizosaccharomyces pombe 972h- (Taxon ID: 284812)
Expression System(s):
Method Details:
Experimental Method:
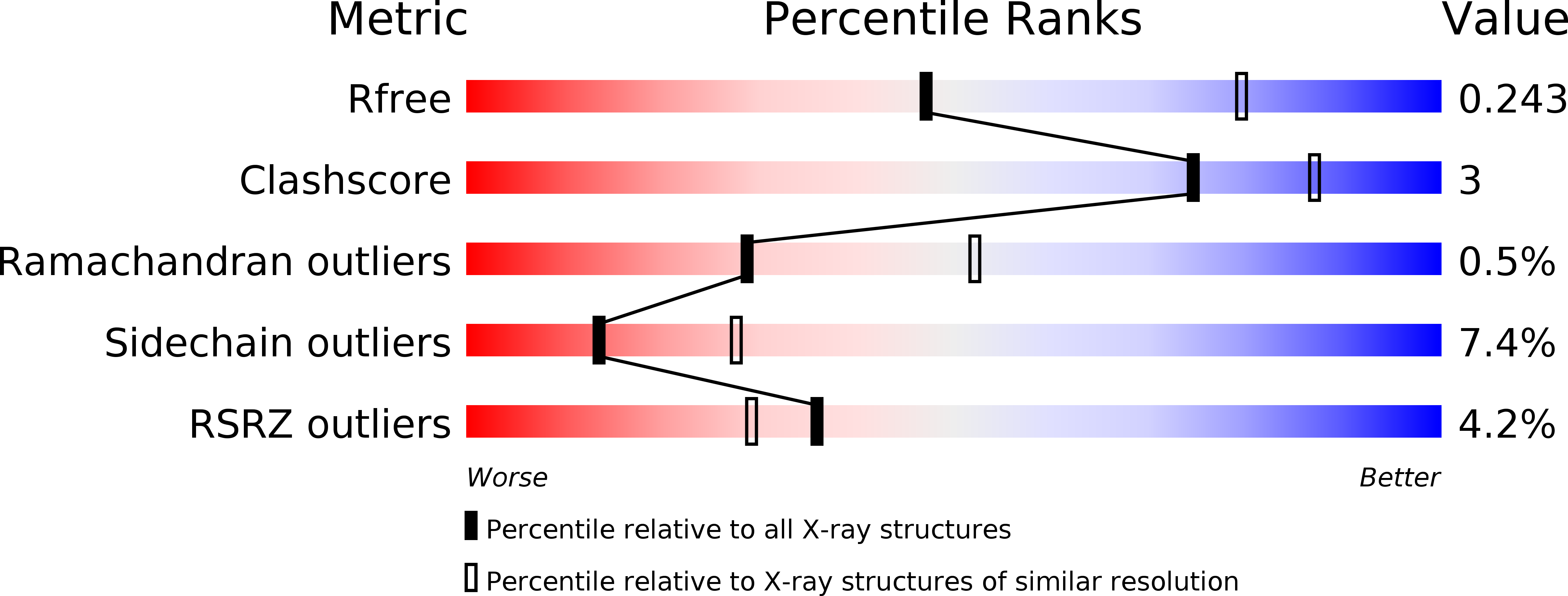
Resolution:
2.60 Å
R-Value Free:
0.23
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 21 21 21