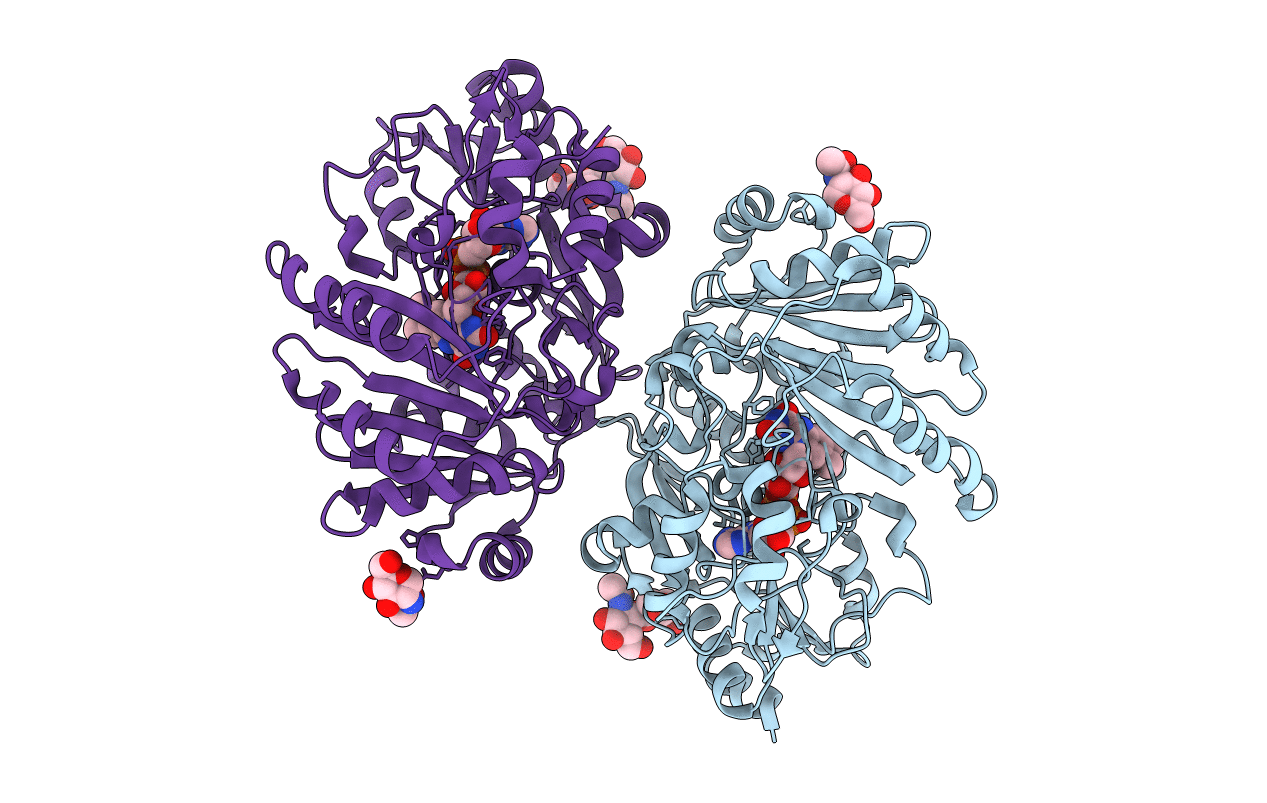
Deposition Date
2012-02-08
Release Date
2012-12-05
Last Version Date
2024-10-30
Entry Detail
PDB ID:
4DNS
Keywords:
Title:
Crystal structure of Bermuda grass isoallergen BG60 provides insight into the various cross-allergenicity of the pollen group 4 allergens
Biological Source:
Source Organism(s):
Cynodon dactylon (Taxon ID: 28909)
Method Details:
Experimental Method:
Resolution:
2.15 Å
R-Value Free:
0.22
R-Value Work:
0.18
R-Value Observed:
0.22
Space Group:
P 43 21 2


