Deposition Date
2012-02-07
Release Date
2012-11-14
Last Version Date
2024-11-27
Entry Detail
PDB ID:
4DMD
Keywords:
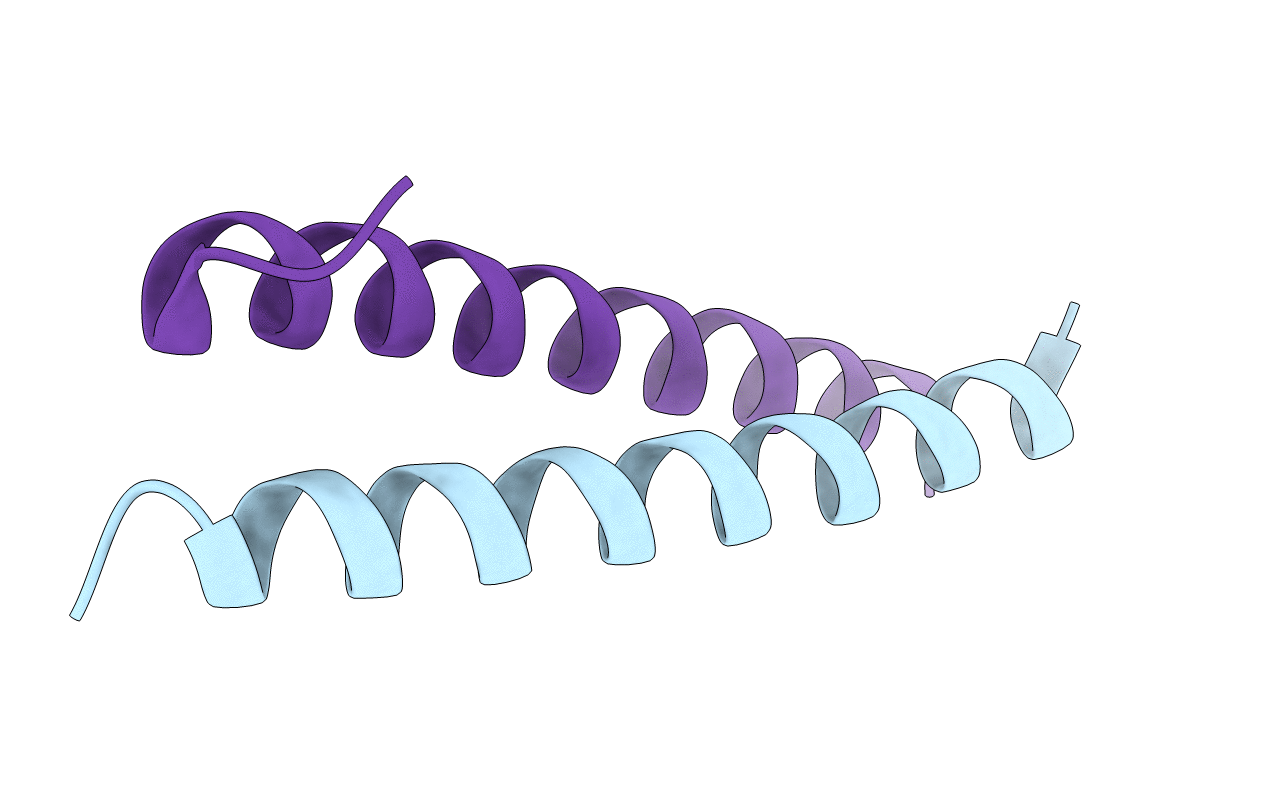
Title:
GCN4 leucine zipper domain in a dimeric oligomerization state
Biological Source:
Source Organism(s):
synthetic construct (Taxon ID: 32630)
Method Details:
Experimental Method:
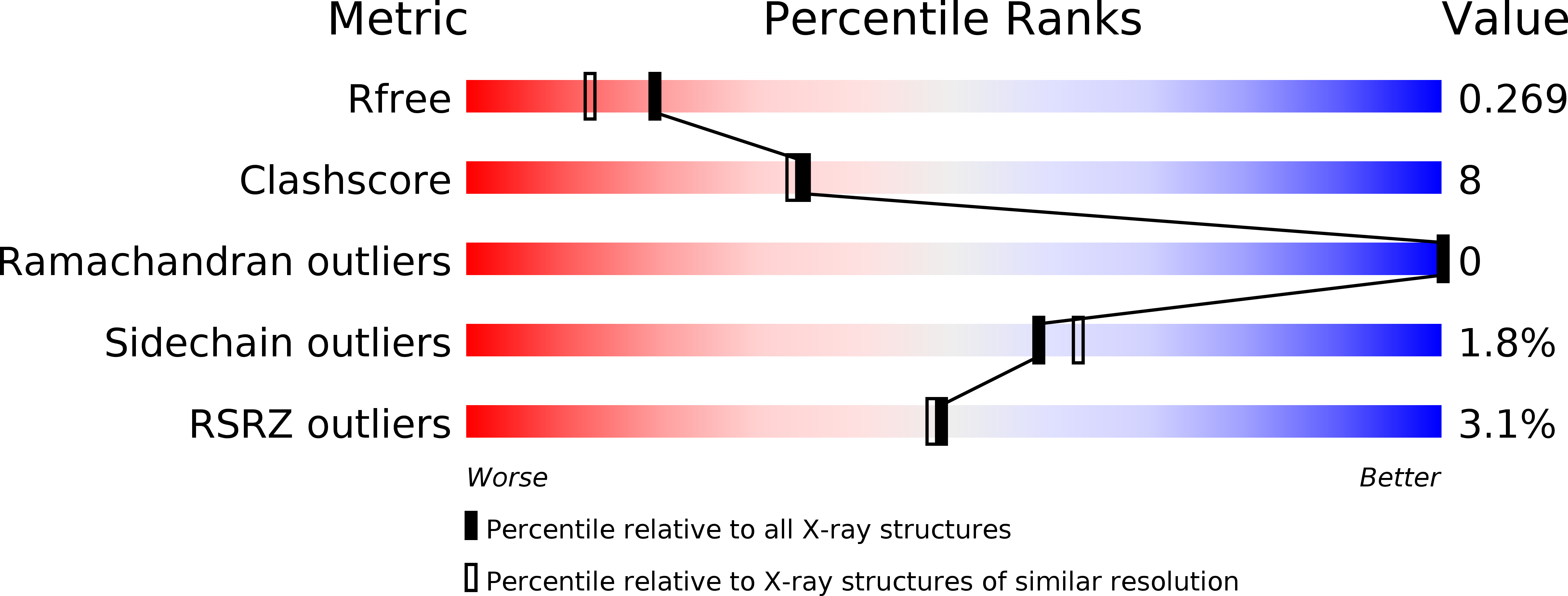
Resolution:
2.00 Å
R-Value Free:
0.26
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
C 1 2 1