Deposition Date
2014-11-27
Release Date
2015-02-11
Last Version Date
2023-12-20
Entry Detail
PDB ID:
4D7S
Keywords:
Title:
Structure of the SthK Carboxy-Terminal Region in complex with cGMP
Biological Source:
Source Organism(s):
SPIROCHAETA THERMOPHILA DSM 6192 (Taxon ID: 665571)
Method Details:
Experimental Method:
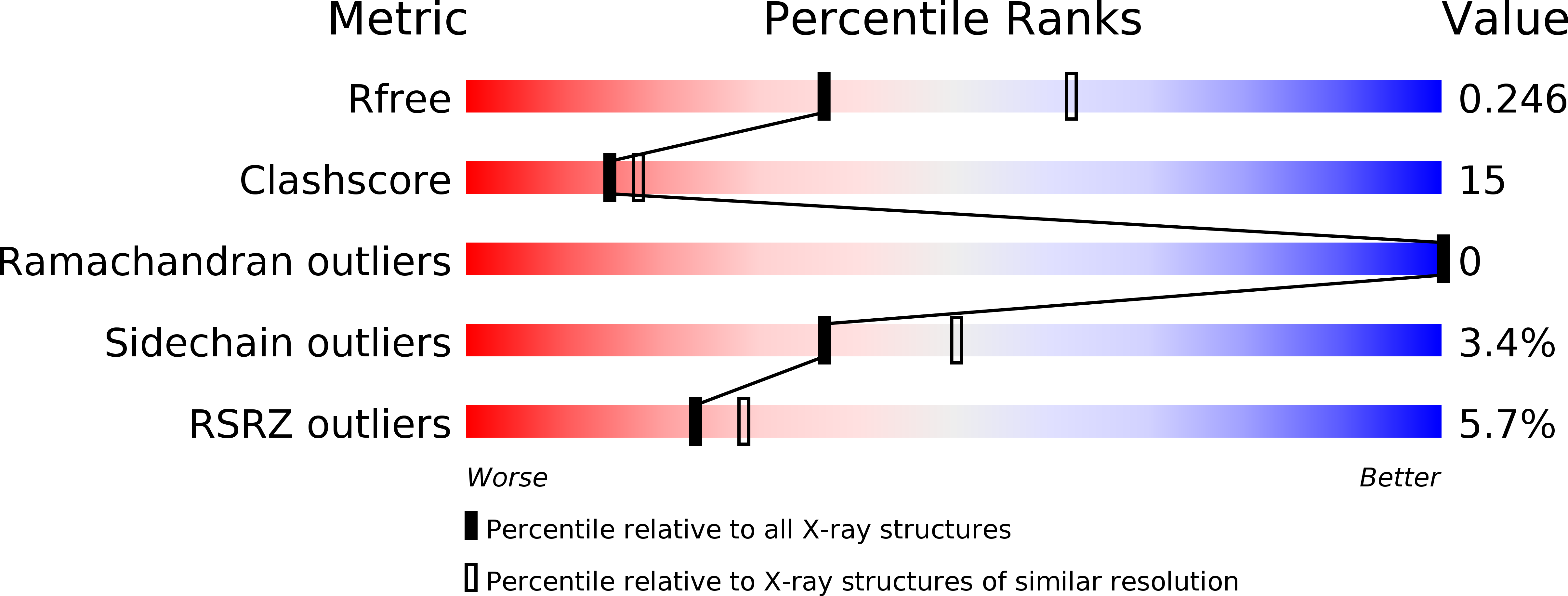
Resolution:
2.55 Å
R-Value Free:
0.24
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
I 4