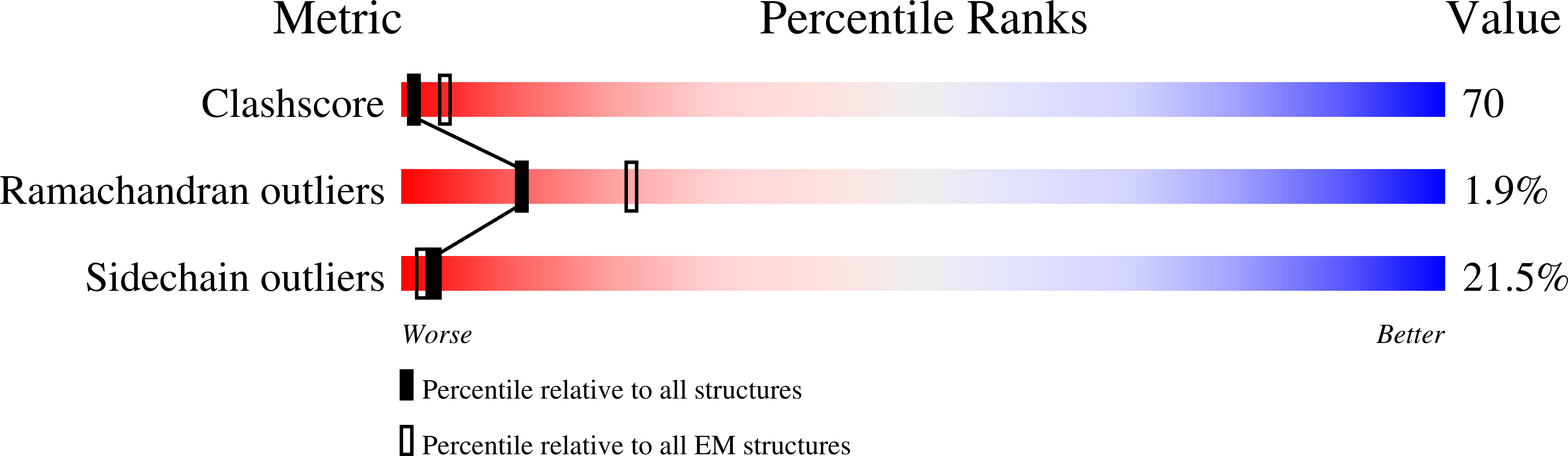
Deposition Date
2014-10-21
Release Date
2014-12-10
Last Version Date
2024-05-08
Entry Detail
PDB ID:
4D3E
Keywords:
Title:
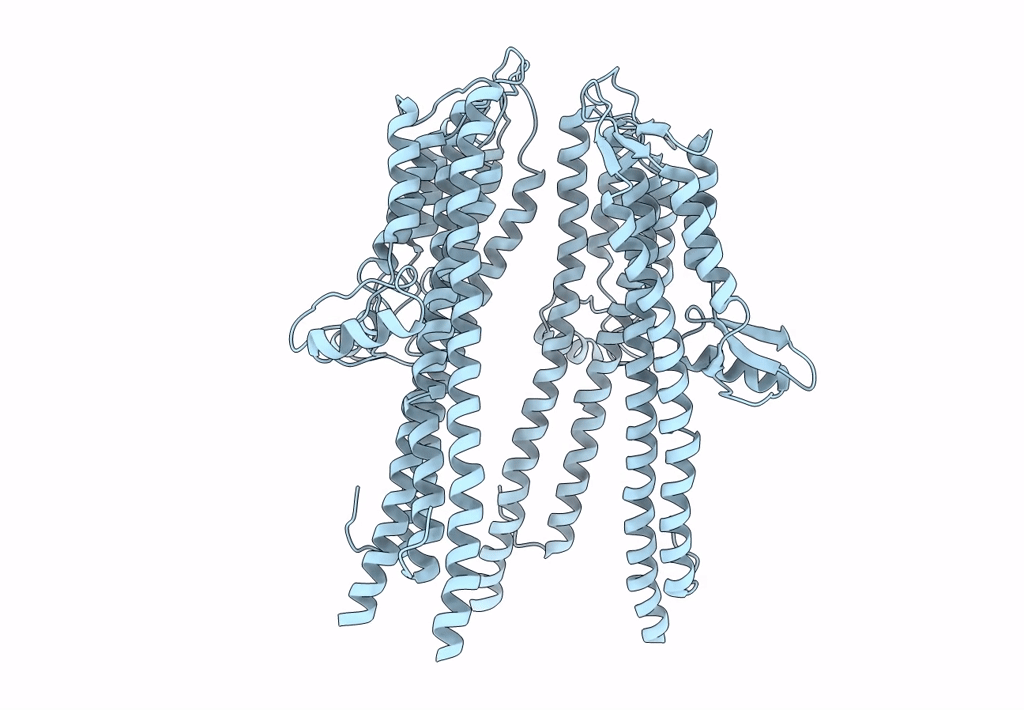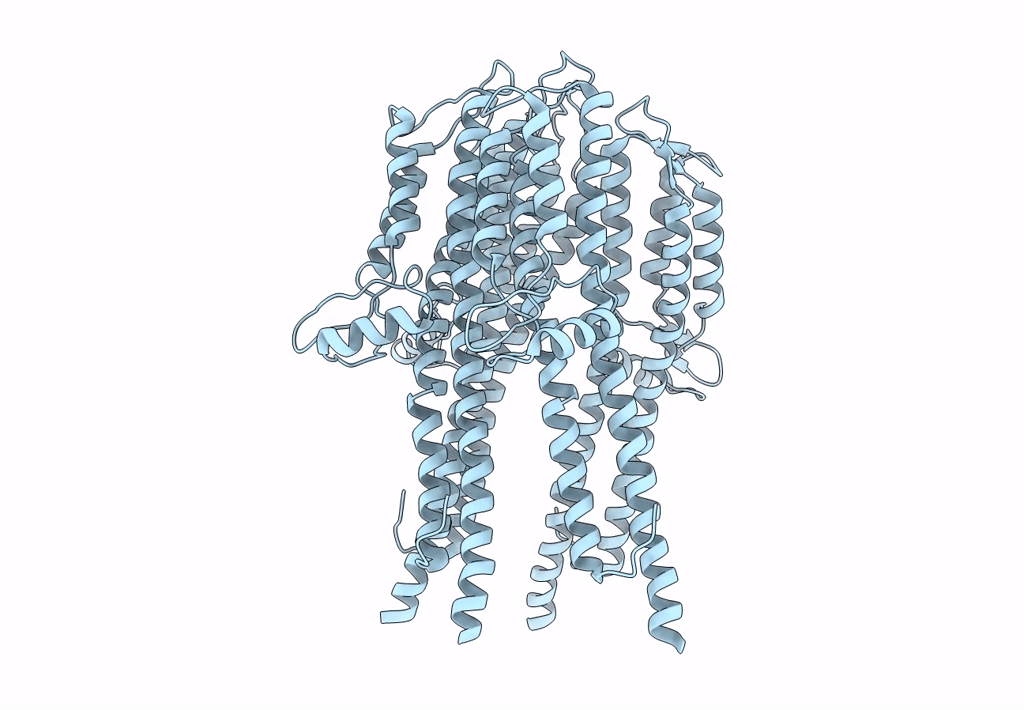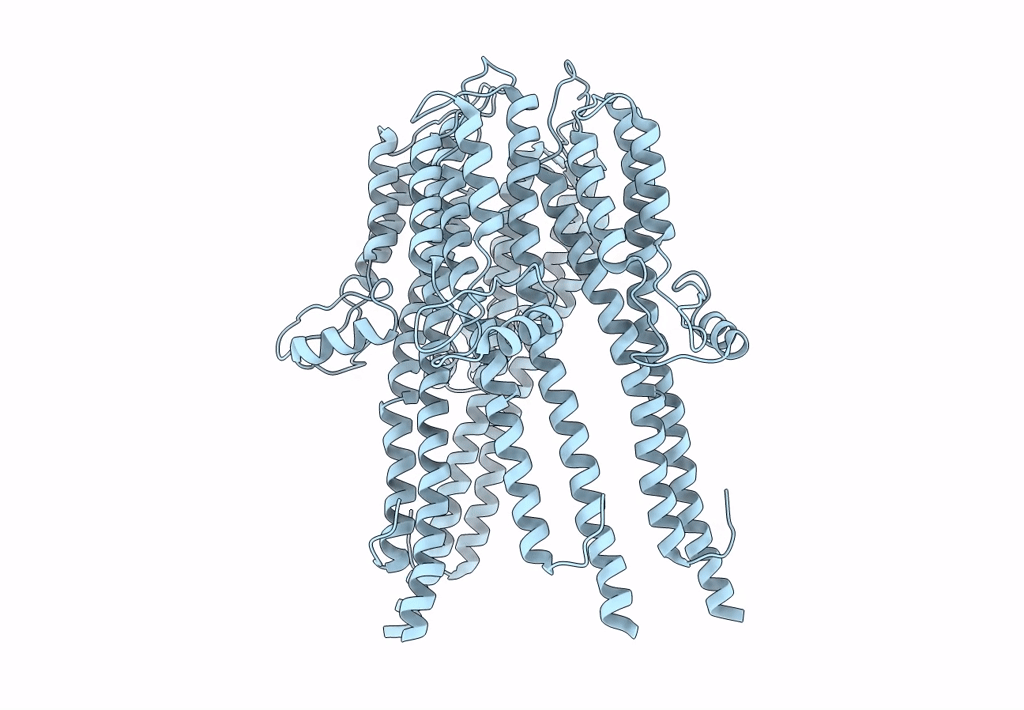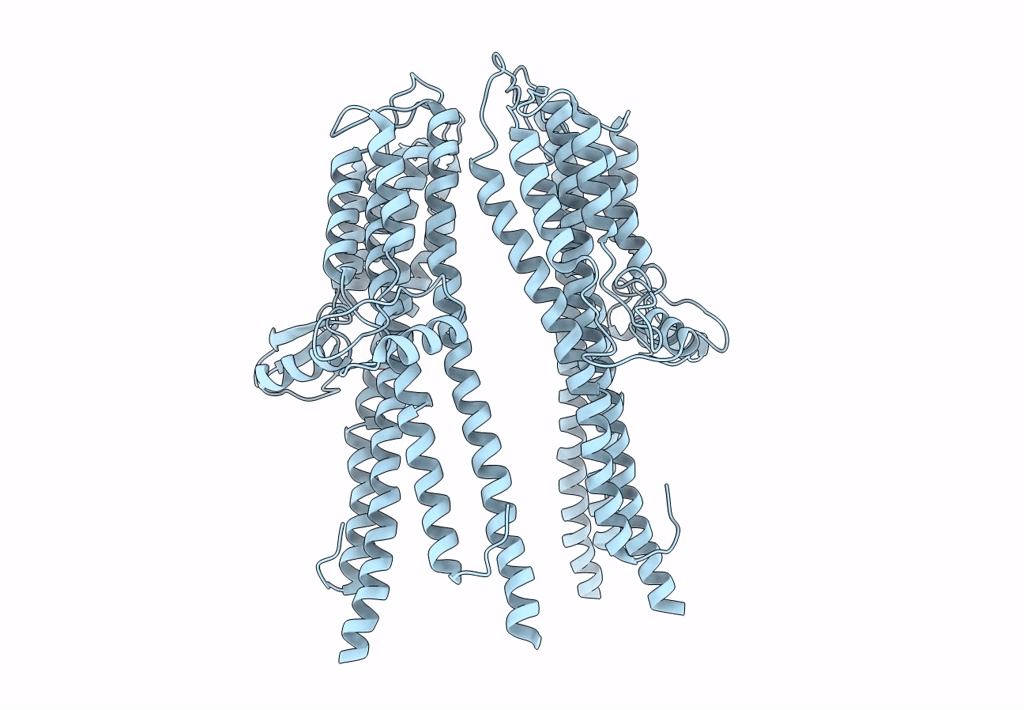
Tetramer of IpaD, modified from 2J0O, fitted into negative stain electron microscopy reconstruction of the wild type tip complex from the type III secretion system of Shigella flexneri
Biological Source:
Source Organism(s):
SHIGELLA FLEXNERI 5A STR. M90T (Taxon ID: 1086030)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
24.00 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE