Deposition Date
2014-02-11
Release Date
2014-03-12
Last Version Date
2023-12-20
Entry Detail
PDB ID:
4CQ4
Keywords:
Title:
C-terminal fragment of Af1503-sol: transmembrane receptor Af1503 from Archaeoglobus fulgidus engineered for solubility
Biological Source:
Source Organism:
ARCHAEOGLOBUS FULGIDUS (Taxon ID: 2234)
Host Organism:
Method Details:
Experimental Method:
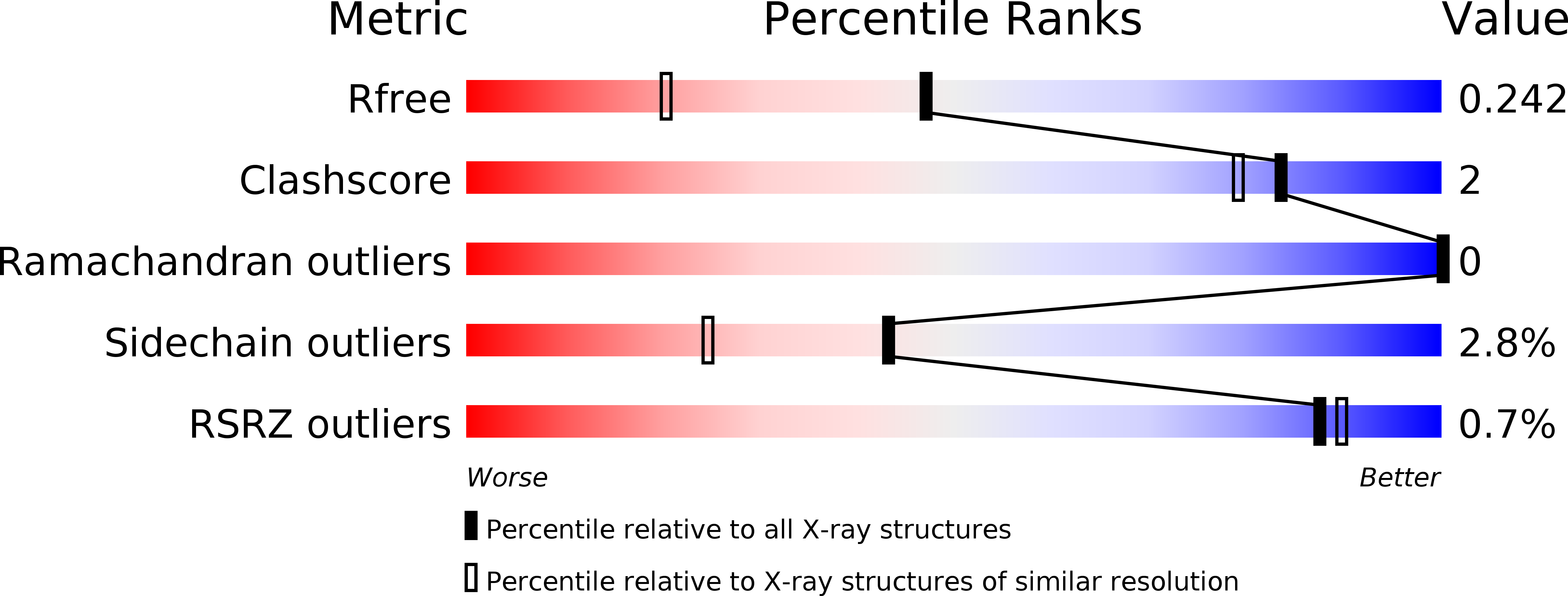
Resolution:
1.70 Å
R-Value Free:
0.24
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 1 21 1