Deposition Date
2014-01-25
Release Date
2015-02-04
Last Version Date
2023-12-20
Entry Detail
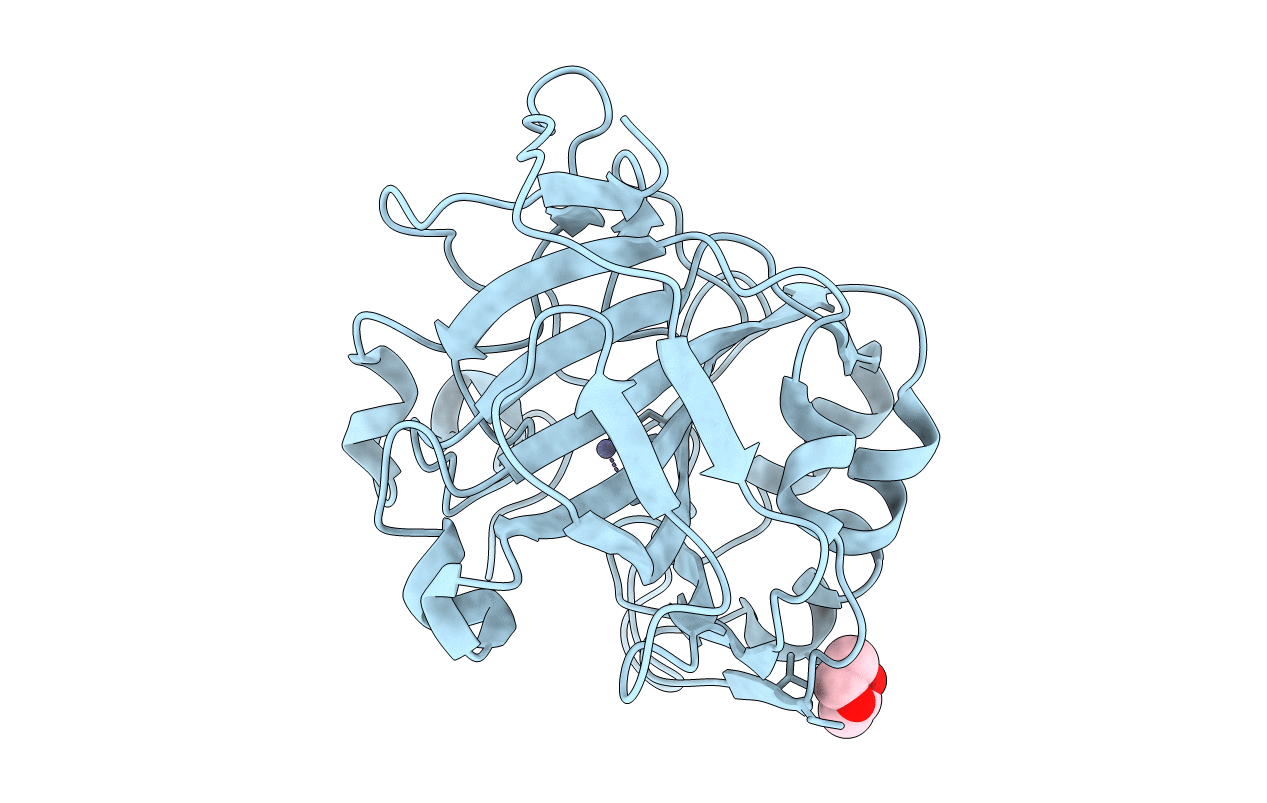
PDB ID:
4CNX
Keywords:
Title:
Surface residue engineering of bovine carbonic anhydrase to an extreme halophilic enzyme for potential application in postcombustion CO2 capture
Biological Source:
Source Organism(s):
BOS TAURUS (Taxon ID: 9913)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
1.23 Å
R-Value Free:
0.14
R-Value Work:
0.11
R-Value Observed:
0.11
Space Group:
P 1 21 1


