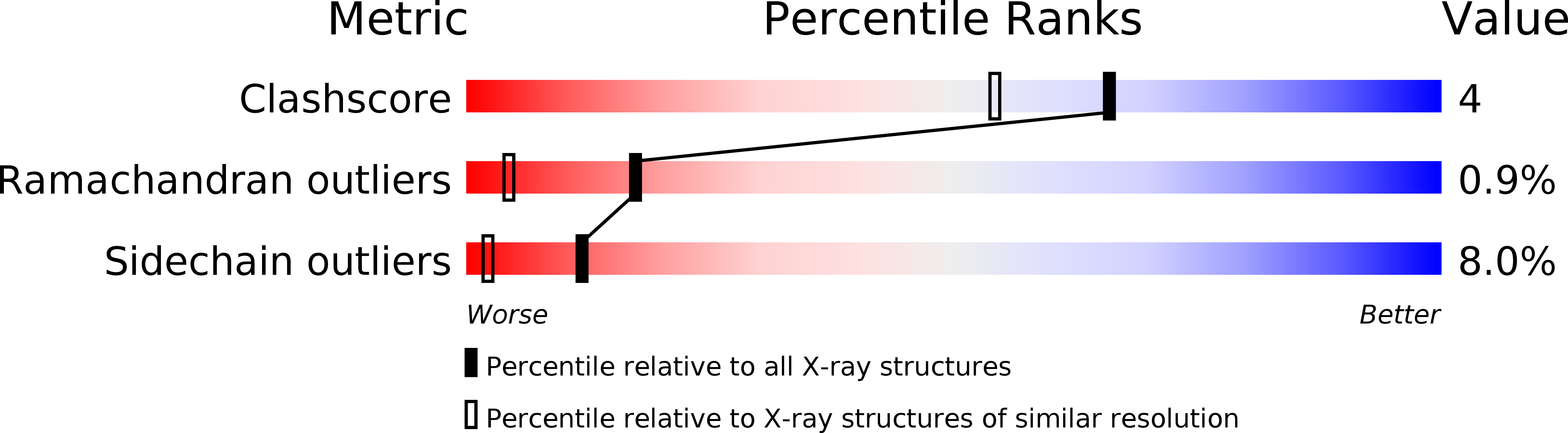
Deposition Date
1984-11-26
Release Date
1985-04-01
Last Version Date
2024-10-23
Entry Detail
PDB ID:
4CHA
Keywords:
Title:
STRUCTURE OF ALPHA-*CHYMOTRYPSIN REFINED AT 1.68 ANGSTROMS RESOLUTION
Biological Source:
Source Organism(s):
Bos taurus (Taxon ID: 9913)
Method Details: