Deposition Date
2013-10-29
Release Date
2014-02-19
Last Version Date
2025-04-09
Entry Detail
PDB ID:
4CCV
Keywords:
Title:
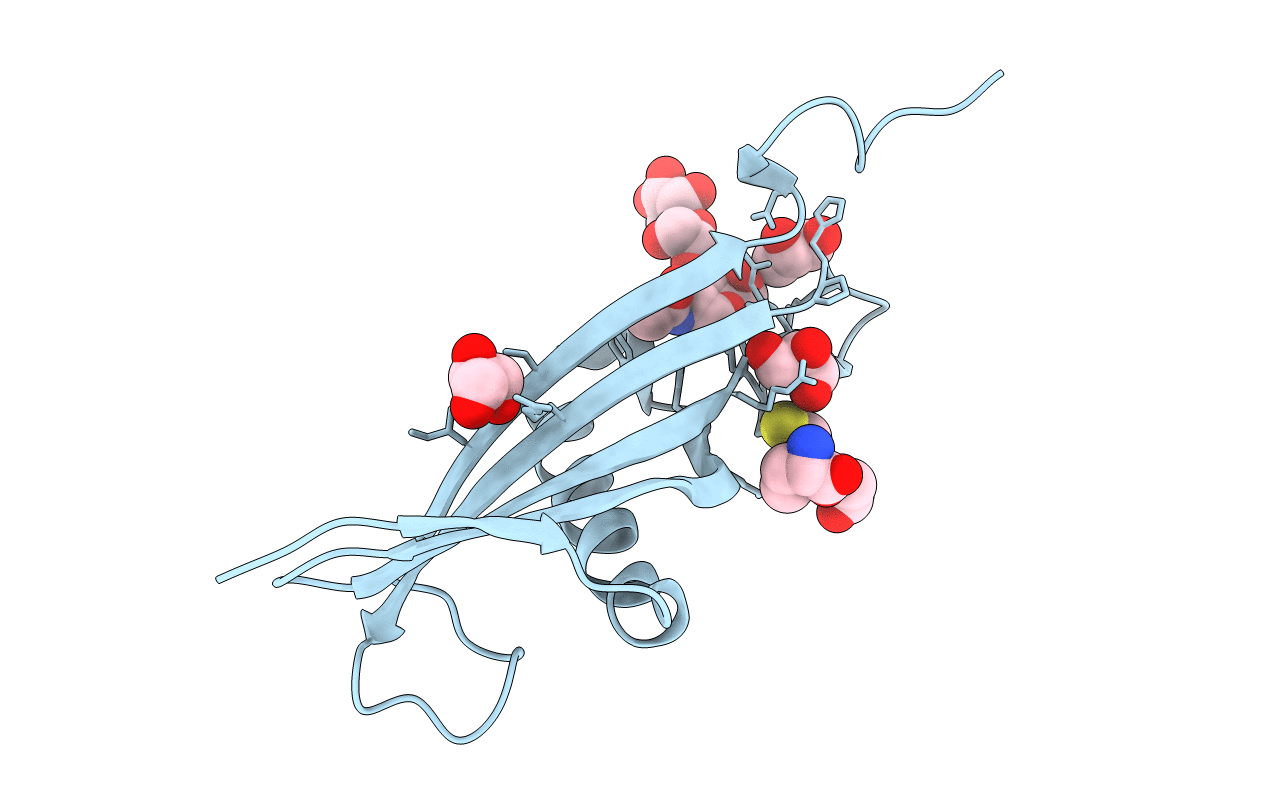
Crystal structure of histidine-rich glycoprotein N2 domain reveals redox activity at an interdomain disulfide bridge: Implications for the regulation of angiogenesis
Biological Source:
Source Organism(s):
ORYCTOLAGUS CUNICULUS (Taxon ID: 9986)
Method Details:
Experimental Method:
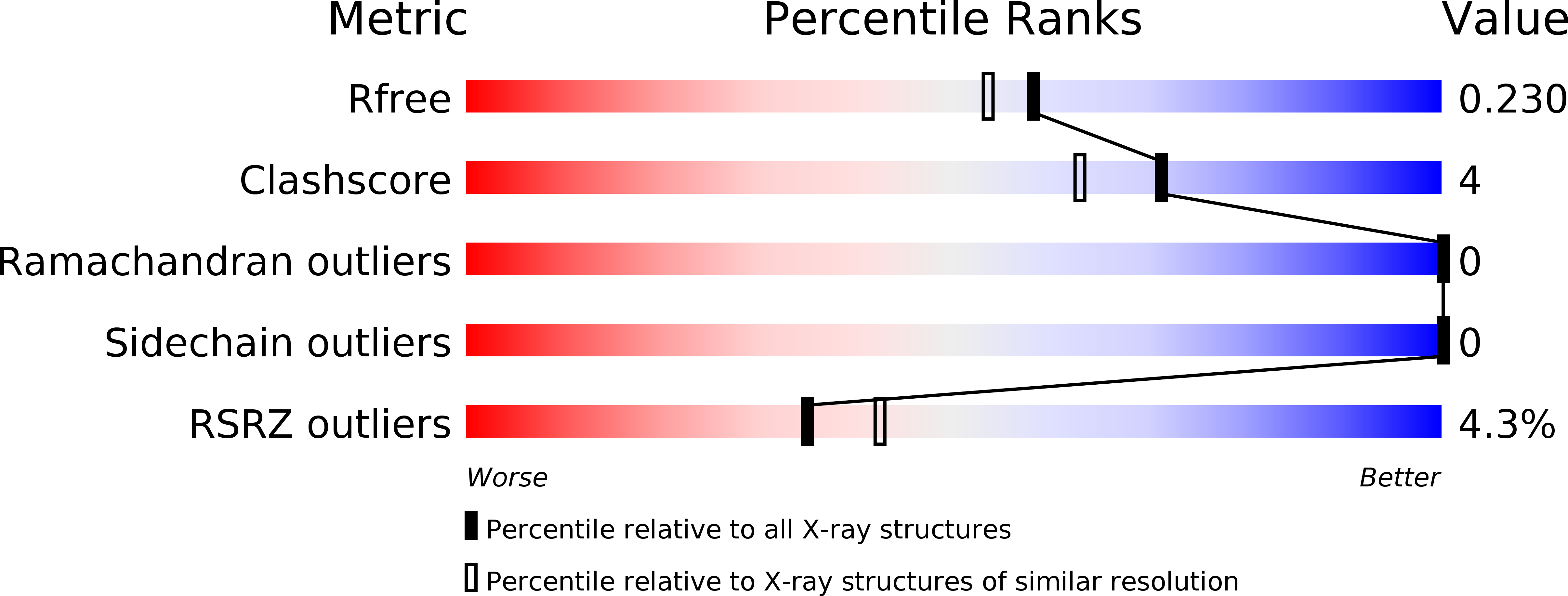
Resolution:
1.93 Å
R-Value Free:
0.22
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
P 31 2 1