Deposition Date
2013-10-03
Release Date
2014-10-22
Last Version Date
2024-11-06
Entry Detail
PDB ID:
4C9T
Keywords:
Title:
BACTERIAL CHALCONE ISOMERASE IN open CONFORMATION FROM EUBACTERIUM RAMULUS AT 2.0 A RESOLUTION, SelenoMet derivative
Biological Source:
Source Organism:
EUBACTERIUM RAMULUS (Taxon ID: 39490)
Host Organism:
Method Details:
Experimental Method:
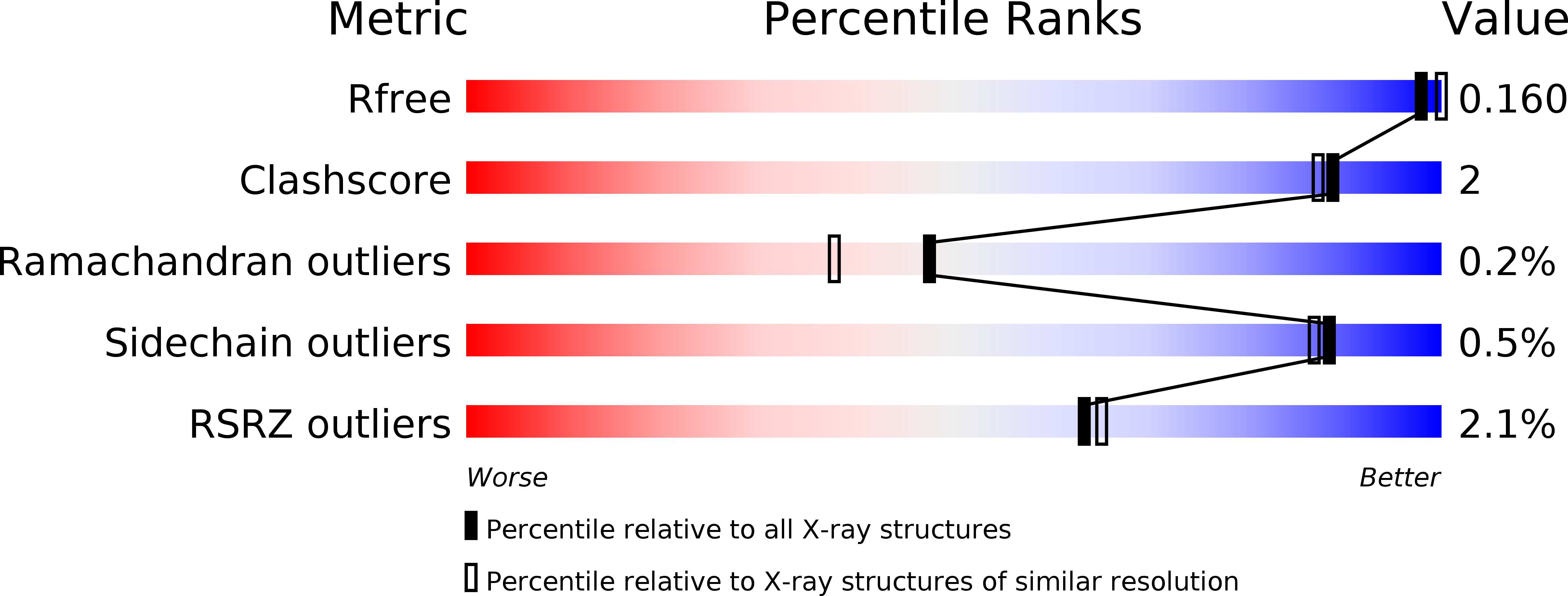
Resolution:
1.98 Å
R-Value Free:
0.15
R-Value Work:
0.13
R-Value Observed:
0.13
Space Group:
I 21 21 21