Deposition Date
2013-09-09
Release Date
2014-02-12
Last Version Date
2023-12-20
Entry Detail
PDB ID:
4C4W
Keywords:
Title:
Structure of a rare, non-standard sequence k-turn bound by L7Ae protein
Biological Source:
Source Organism(s):
HOMO SAPIENS (Taxon ID: 9606)
ARCHAEOGLOBUS FULGIDUS (Taxon ID: 224325)
KNEALLHAZIA SOLENOPSAE (Taxon ID: 694064)
ARCHAEOGLOBUS FULGIDUS (Taxon ID: 224325)
KNEALLHAZIA SOLENOPSAE (Taxon ID: 694064)
Expression System(s):
Method Details:
Experimental Method:
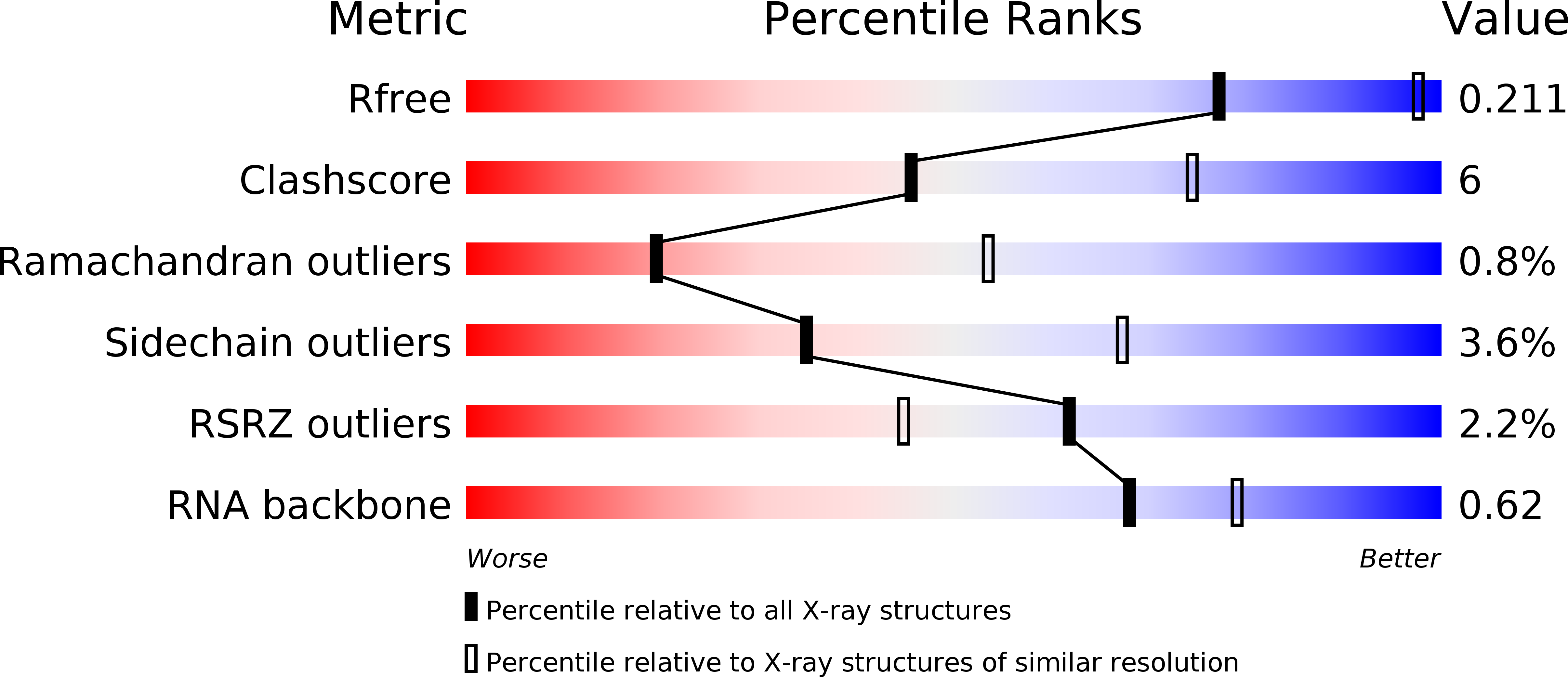
Resolution:
2.95 Å
R-Value Free:
0.21
R-Value Work:
0.16
R-Value Observed:
0.16
Space Group:
C 2 2 21